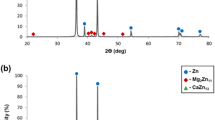
Abstract
The in vitro corrosion behavior and biocompatibility of two Zr alloys, Zr-2.5Nb, employed for the manufacture of CANDU reactor pressure tubes, and Zr-1.5Nb-1Ta (at%), for use as implant materials have been assessed and compared with those of Grade 2 Ti, which is known to be a highly compatible metallic biomaterial. The in vitro corrosion resistance was investigated by open circuit potential and electrochemical impedance spectroscopy (EIS) measurements, as a function of exposure time to an artificial physiological environment (Ringer’s solution). Open circuit potential values indicated that both the Zr alloys and Grade 2 Ti undergo spontaneous passivation due to spontaneously formed oxide film passivating the metallic surface, in the aggressive environment. It also indicated that the tendency for the formation of a spontaneous oxide is greater for the Zr-1.5Nb-1Ta alloy and that this oxide has better corrosion protection characteristics than the ones formed on Grade 2 Ti or on the Zr-2.5Nb alloy. EIS study showed high impedance values for all samples, increasing with exposure time, indicating an improvement in corrosion resistance of the spontaneous oxide film. The fit obtained suggests a single passive film presents on the metals surface, improving their resistance with exposure time, presenting the highest values to the Zr-1.5Nb-1Ta alloy. For the biocompatibility analysis human osteosarcoma cell line (Saos-2) and human primary bone marrow stromal cells (BMSC) were used. Biocompatibility tests showed that Saos-2 cells grow rapidly, independently of the surface, due to reduced dependency from matrix deposition and microenvironment recognition. BMSC instead display a reduced proliferation, possibly caused by a reduced crosstalk with the metal surface microenvironment. However, once the substrate has been colonized, BMSC seem to respond properly to osteoinduction stimuli, thus supporting a substantial equivalence in the biocompatibility among the Zr alloys and Grade 2 titanium. In summary, high in vitro corrosion resistance together with satisfactory biocompatibility make the Zr-2.5Nb and Zr-1.5Nb-1Ta crystalline alloys promising biomaterials for surgical implants.







Similar content being viewed by others
References
Lohrengel MM. Formation of ionic space charge layers in oxide films on valve metals. Electrochim Acta. 1994;39:1265.
Malik F. A study of passive films on valve metals. Thin Solid Films. 1991;206:345.
Bockris JOM. Modern aspect of electrochemistry, vol. 14. New York: Plenum Press; 1982.
Badawy WA, Felske A, Plieth WJ. Electrochemical, photoelectrochemical behaviour of passivated Ti, Nb electrodes in nitric acid solutions. Electrochim Acta. 1989;34:1711.
Biaggio SR, Rocha-Filho RC, Vilche JR, Varela FE, Gassa LM. A study of thin anodic WO3 films by electrochemical impedance spectroscopy. Electrochim Acta. 1997;42:1751.
Marino CEB, Oliveira EM, Rocha-Filho RC, Biaggio SR. On the stability of thin-anodic-oxide films of titanium in acid phosphoric media. Corros Sci. 2001;43:1465.
Salot R, Lefevbre-Joud F, Baroux B. Electrochemical behavior of thin anodic oxide films on Zircaloy-4. J Electrochem Soc. 1996;143:3902.
Hammad AM, El-Mashri SM, Nasr MA. Mechanical properties of the Zr-1% Nb alloy at elevated temperatures. J Nuclear Mater. 1992;186:166.
Oliveira NTC, Biaggio SR, Rocha-Filho RC, Bocchi N. Studies on the stability of anodic oxides on zirconium biocompatible alloys. J Braz Chem Soc. 2002;13:463.
Pourbaix M. Electrochemical corrosion of metallic biomaterials. Biomaterials. 1984;5:122.
Biehl V, Breme J. Metallic biomaterials. Mat-wiss u Werkstoff-tech. 2001;32:137.
Niinomi M, Kuroda D, Fukunaga K, Morinaga M, Kato Y, Yashiro T, Suzuki A. Corrosion wear fracture of new β type biomedical titanium alloys. Mater Sci Eng A. 1999;263:193.
Okazaki Y, Rao S, Tateishi T, Ito Y. Cytocompatibility of various metals and development of new titanium alloys for medical implants. Mater Sci Eng A. 1998;243:250.
Bjursten LM, Emanuelsson L, Ericson LE, Thomsen P, Lausmaa J, Mattson L, Rolander U, Kasemo B. Method for ultrastructural studies of the intact tissue-metal interface. Biomaterials. 1990;11:596.
Thompsen P, Larsson C, Ericson LE, Sennerby L, Lausmaa J, Kasemo B. Structure of the interface between rabbit cortical bone and implants of gold, zirconium and titanium. J Mater Sci: Mater Med. 1997;8:653.
Sherepo KM, Red’ko IA. Use of zirconium for implants in traumatology and orthopedics. Med Tekh. 2004;2:22.
Cabrini RL, Guglielmotti MB, Almagro JC. Histomorphometry of initial bone healing around zirconium implants in rats. Implant Dent. 1993;2:264.
Guglielmotti MB, Cabrini RL, Guerrero C. Chronodynamic evaluation of the stages of osseointegration in zirconium laminar implants. Acta Odontol Latinoam. 1997;10:11.
Guglielmotti MB, Cabrini RL, Renou S. A histomorphometric study of tissue interface by laminar test in rats. Int J Oral Maxillofac Implants. 1999;14:565.
Kulokaov OB, Doktorov AA, D’iakova SV, Denisov-Nikol’skii IuI, Grotz KA. Experimental study of osseointegration of zirconium and titanium dental implants. Morfologiia. 2005;127:52.
Cox B. Advances in corrosion science and technology, vol. 5. New York: Plenum Press; 1976.
Yun YH, Turitto VT, Daigle KP, Kovacs P, Davidson JA, Slack SM. Initial, hemocompatibility studies of titanium and zirconium alloys: Prekallikrein activation, fibrinogen adsorption, and their correlation with surface electrochemical properties. J Biomed Mater Res. 1996;32:77.
Helsen JA, Breme J. Metals as biomaterials. Chichester, England: Wiley; 1998.
Matsuno H, Yokoyama A, Watary F, Uo M, Kawasaki T. Biocompatibility and osteogenesis of refractory metal implants, titanium, hafnium, niobium, tantalum and rhenium. Biomaterials. 2001;22:1253.
Gerardi S. ASTM Handbook, vol. 2. Metals Park: ASM International; 1993.
Eisenbarth E, Velten D, Müller M, Thull R, Breme J. Biocompatibility of β-stabilizing elements of titanium alloys. Biomaterials. 2004;25:5705.
Branzoi IV, Iordoc M, Codescu M. Electrochemical studies on the stability and corrosion resistance of new zirconium-based alloys for biomedical applications. Surf Interface Anal. 2008;40:167.
Yto A, Okazaki Y, Tateishi T, Ito Y. Mechanical properties of the binary titanium-zirconium alloys and their potential for biomedical materials. J Biomed Mater Res. 1995;29:943.
Khan MA, Williams RL, Williams DF. In vitro corrosion and wear of titanium alloys in the biological environment. Biomaterials. 1996;17:2117.
Rosalbino F, Macciò D, Saccone A, Angelini E, Delfino S. Effect of Nb alloying additions on the characteristics of anodic oxide films on zirconium and their stability in NaOH solution. J Solid State Electrochem. 2010;14:1451.
Rodan SB, Imai Y, Thiede MA, Wesolowski G, Thompson D, Bar-Shavit Z, Shull S, Mann K, Rodan GA. Characterization of a human osteosarcoma cell line (Saos-2) with osteoblastic properties. Cancer Res. 1987;47:4961.
Giannoni P, Muraglia A, Giordano C, Narcisi R, Cancedda R, Quarto R, Chiesa R. Osteogenic differentiation of human mesenchymal stromal cells on surface-modified titanium alloys for orthopedic and dental implants. Int J Artif Organs. 2009;32:811.
Muraglia A, Cancedda R, Quarto R. Clonal mesenchymal progenitors from human bone marrow differentiate in vitro according to a hierarchical model. J Cell Sci. 2000;113:1161.
Zerega B, Cermelli S, Bianco P, Cancedda R, Cancedda FD. Parathyroid hormone [PTH(1–34)] and parathyroid hormone-related protein [PTHrP(1–34)] promote reversion of hypertrophic chondrocytes to a prehypertrophic proliferating phenotype and prevent terminal differentiation of osteoblast-like cells. J Bone Miner Res. 1999;14(8):1281.
Abriata JP, Bolcich JC. The Nb–Zr (Niobium–Zirconium) system. Bull Alloy Phase Diag. 1982;3(1):34.
Villars P, Prince A, Okamoto H. Handbook of ternary alloy phase diagrams. Metals Park, Ohio: ASM International; 1995.
Metikos-Hukovic M, Kwokal A, Piljac J. The influence of niobium and vanadium on passivity of titanium-based implants in physiological solution. Biomaterials. 2003;24:3765.
Oliveira NTC, Aleixo G, Caram R, Guastaldi AC. Development of Ti–Mo alloys for biomedical applications: microstructure and electrochemical characterization. J Mater Sci Eng A. 2007;452–453:727.
Temiselvi S, Raman V, Rajendran N. Corrosion behaviour of Ti-6Al-7Nb and Ti-6Al-4 V ELI alloys in the simulated body fluid solution by electrochemical impedance spectroscopy. Electrochim Acta. 2006;52:839.
Gonzalez JEG, Mirza-Rosca JC. Study of the corrosion behaviour of titanium and some of its alloys for biomedical and dental implant applications. J Electroanal Chem. 1999;471:109.
Shukla AK, Balasubramaniam R. Effect of surface treatment on electrochemical behavior of CP Ti, Ti-6Al-4 V and Ti-13Nb-13Zr alloys in simulated human body fluid. Corros Sci. 2006;48:1696.
Oliveira NTC, Biaggio SR, Piazza S, Sunseri C, Di Quarto F. Photo-electrochemical and impedance investigation of passive layers grown anodically on titanium alloys. Electrochim Acta. 2004;49:4563.
Martins DQ, Osório WR, Souza MEP, Caram R, Garcia A. Effect of Zr content on microstructure and corrosion resistance of Ti-30Nb-Zr casting alloys for biomedical applications. Electrochim Acta. 2008;53:2809.
Macdonald JR. Impedance spectroscopy. New York: Wiley; 1987.
Mackie EJ, Ramsey SJ. Modulation of osteoblast behaviour by tenascin. Cell Sci. 1996;106:1597.
Haynesworth SE, Goshima J, Goldberg VM, Caplan AI. Characterization of cells with osteogenic potential from human marrow. Bone. 1992;13(1):81.
Braccini A, Wendt D, Jaquiery C, Jakob M, Heberer M, Kenins L, Wodnar-Filpowicz A, Quarto R, Martin I. Three-dimensional perfusion culture of human bone marrow cells and generation of osteoinductive grafts. Stem Cells. 2005;23:1066.
Giannoni P, Mastrogiacomo M, Alini M, Pearce SG, Corsi A, Santolini F, Muraglia A, Bianco P, Cancedda R. Regeneration of large bone defects in sheep using bone marrow stromal cells. J Tissue Eng Regen Med. 2008;2:253.
Zreiqat H, Howlett CR. Titanium substrata composition influences osteoblastic phenotype: In vitro study. J Biomed Mater Res. 1999;47:360.
Marom R, Shur I, Solomon R, Benayahu D. Characterization of adhesion and differentiation markers of osteogenic marrow stromal cells. J Cell Physiol. 2005;202:41.
Ganss B, Kim RH, Sodek J. Bone sialoprotein. Crit Rev Oral Biol Med. 1999;10:79.
Roca H, Phimphilai M, Gopalakrishnan R, Xiao G, Franceschi RT. Cooperative interactions between RUNX2 and homeodomain protein-binding sites are critical for the osteoblast-specific expression of the bone sialoprotein gene. J Biol Chem. 2005;280:30845.
Carvalho RS, Bumann A, Schaffer JL, Gerstenfeld LC. Predominant integrin ligands expressed by osteoblasts show preferential regulation in response to both cell adhesion and mechanical perturbation. J Cell Biochem. 2002;84:497.
Lamour V, Detry C, Sanchez C, Henrotin Y, Castronovo V, Bellahcene A. Runx2- and histone deacetylase 3-mediated repression is relieved in differentiating human osteoblast cells to allow high bone sialoprotein expression. J Biol Chem. 2007;282:36240.
Behonick DJ, Xing Z, Lieu S, Buckley JM, Lotz JC, Marcucio RS, Werb Z, Miclau T, Colnot C. Role of matrix metalloproteinase 13 in both endochondral and intramembranous ossification during skeletal regeneration. PLoS ONE. 2007;2:e1150.
Park JW, Kim YJ, Jang JH. Enhanced osteoblast response to hydrophilic strontium and/or phosphate ions-incorporated titanium oxide surfaces. Clin Oral Implants Res. 2010;21(4):398.
Balloni S, Calvi EM, Damiani F, Bistoni G, Calvitti M, Locci P, Becchetti E, Marinucci L. Effects of titanium surface roughness on mesenchymal stem cell commitment and differentiation signaling. Int J Oral Maxillofac Implants. 2009;24(4):627.
Chung JW, Kim MS, Piao ZH, Jeong M, Yoon SR, Shin N, Kim SY, Hwang ES, Yang Y, Lee YH, Kim YS, Choi I. Osteopontin promotes the development of natural killer cells from hematopoietic stem cells. Stem Cells. 2008;26(8):2114.
Pham QP, Kasper FK, Scott Baggett L, Raphael RM, Jansen JA, Mikos AG. The influence of an in vitro generated bone-like extracellular matrix on osteoblastic gene expression of marrow stromal cells. Biomaterials. 2008;29(18):2729.
Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, Tagliafico E, Ferrari S, Gheron Robey P, Riminucci M, Bianco P. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rosalbino, F., Macciò, D., Giannoni, P. et al. Study of the in vitro corrosion behavior and biocompatibility of Zr-2.5Nb and Zr-1.5Nb-1Ta (at%) crystalline alloys. J Mater Sci: Mater Med 22, 1293–1302 (2011). https://doi.org/10.1007/s10856-011-4301-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-011-4301-z