Abstract
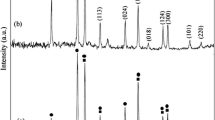
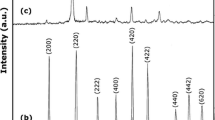
In this investigation, a two-step sol–gel method was developed to prepare CoTiO3 nanostructures by using cobalt(II) acetate and tetra-n-butyl titanate (TNBT). The type of solvent and polymeric agent as well as calcination temperature and the molar ratio of TNBT to 1,3,5-benzenetricarboxylic acid (trimesic acid) were the studied parameters. Based on the XRD results, it was found that pure CoTiO3 nanostructures were produced only in presence of ethanol and trimesic acid. In the present study, trimesic acid has been used effectively to synthesize pure phase of CoTiO3 which avoids the oxidation of Co2+ as well as the reduction of Ti4+. This work provides a general, simple, and effective method to control the structure and morphology of CoTiO3 in ethanol solution, which will be important in inorganic synthesis methodology. The products were characterized by XRD, SEM, FT-IR, TEM, DRS, and EDS.











Similar content being viewed by others
References
S. M. Hosseinpoor-Mashkani, F. Mohandes, M. Salavati-Niasari, and K. Venkateswara-Rao (2012). Mater. Res. Bull. 47, 3148.
M. Zahedifar, Z. Chamanzadeh, and S. M. Hosseinpoor-Mashkani (2013). J. Lumin. 135, 66.
S. M. Hosseinpoor-Mashkani, M. Salavati-Niasari, F. Mohandes, and K. Venkateswara-Rao (2013). Mater. Sci. Semicond. Process. 16, 390.
D. Ma, J. Zhao, Y. Li, X. Su, S. Hou, Y. Zhao, X. L. Hao, and L. Li (2010). Colloids Surf. Physicochem. Eng. Asp. 368, 105.
S. Alikhanzadeh-Arani, M. Salavati-Niasari, and M. Almasi-Kashi (2013). Phys. C. 488, 30.
O. Amiri, M. Salavati-Niasari, S. M. Hosseinpour-Mashkani, A. Rafiei, and S. Bagheri (2014). Mater. Sci. Semicond. Process. 27, 261.
S. M. Hosseinpour-Mashkani, M. Salavati-Niasari, and F. Mohandes (2014). Ind. Eng. Chem. 20, 3800.
A. Sobhani, M. Salavati-Niasari, and S. M. Hosseinpour-Mashkani (2012). J. Clust. Sci. 23, 1143.
O. Yamamoto, Y. Takeda, R. Kanno, and M. Noda (1987). Solid State Ion. 22, 241.
H. Obayashi, Y. Sakurai, and T. Gejo (1976). J. Solid State Chem. 17, 299.
Y. Shimizu, K. Uemura, N. Miura, and N. Yamzoe (1988). Chem. Lett. 67, 1979.
T. Ivanova, A. Harizanova, M. Surtchev, and Z. Nenova (2003). Sol. Energy Mater. Sol. Cell. 76, 591.
Y. M. Chiang, D. Birnie, and W. D. Kingery Physical Ceramics (John Wiley & Sons Inc., New York, 1996). 34.
F. Schoofs, M. Egilmez, T. Fix, J. L. MacManus-Driscoll, and M. G. Blamire (2013). J. Magn. Magn. Mater. 332, 67.
X. Chu, X. Liu, G. Wang, and G. Meng (1999). Mater. Res. Bull. 34, 1789.
M. Siemons and U. Simon (2006). Sens. Actuat. B chem. 120, 110.
G. Radnoczi, P. B. Barna, M. Adamik, Z. Czigany, J. Ariake, N. Honda, and K. Ouchi (2000). Cryst. Res. Technol. 35, 707.
T. Kazuyuki, U. Yasuo, T. Shuji, I. Takashi, and U. Akifumi (1984). J. Am. Chem. Soc. 106, 5172.
Y. Brik, M. Kacimi, M. Ziyad, and F. Bozon-Verduraz (2001). J. Catal. 202, 118.
Y. Teraoka, H. Kakebayashi, I. Moriguchi, and S. Kagawa (1991). Chem. Lett. 88, 673.
M. Siemons and U. Simon (2007). Sens. Actuators B 126, 595.
S. H. Chuang, R. H. Gao, D. Y. Wang, H. P. Liu, L. M. Chen, and M. Y. Chiang (2010). J. Chin. Chem. Soc. 57, 932.
H. Y. He (2008). Powder Metall. 51, 224.
Y. J. Lin, Y. H. Chang, W. D. Yang, and B. S. Tsai (2006). J. Non-Cryst. Solids 352, 789.
M. Enhessari, A. Parviz, K. Ozaee, and E. Karamali (2010). J. Exp. Nanosci. 5, 61.
M. Ranjbar, M. Salavati-Niasari, S. M. Hosseinpour-Mashkani, and K. Venkateswara-Rao (2012). Inorg. Organomet. Polym. Mater. 22, 1122.
T. Gholami, M. Salavati-Niasari, M. Bazarganipour, and E. Noori (2013). Superlattices Microstruct. 61, 33.
M. Salavati-Niasari, B. Shoshtari-Yeganeh, and F. Mohandes (2013). Mater. Res. Bull. 48, 1745.
M. Ranjbar, M. Salavati-Niasari, and S. M. Hosseinpour-Mashkani (2012). J. Inorg. Organomet. Poly. Mater. 22, 1093.
J. Luo, X. Xing, R. Yu, Q. Xing, D. Zhang, and X. Chen (2005). J. Alloy. Compd. 402, 263.
J. L. Wang, Y. Q. Li, Y. J. Byon, S. G. Mei, and G. L. Zhang (2013). Powder Technol. 235, 303.
Acknowledgments
Authors are grateful to council of University of Kashan for providing financial support to undertake this work by Grant No (159271/199).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sobhani-Nasab, A., Hosseinpour-Mashkani, S.M., Salavati-Niasari, M. et al. Controlled Synthesis of CoTiO3 Nanostructures Via Two-Step Sol–Gel Method in the Presence of 1,3,5-Benzenetricarboxylic Acid. J Clust Sci 26, 1305–1318 (2015). https://doi.org/10.1007/s10876-014-0814-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-014-0814-1