Abstract
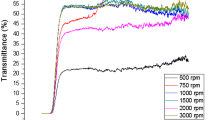
Zinc oxide (ZnO) nanorods were successfully synthesized via a novel hydrothermal route using new set of starting reagents including Zn(OAc)2·2H2O, ethylenediamine and hydrazine. The as-synthesized products were characterized by techniques including X-ray diffraction, energy dispersive spectrometry, Scanning electron microscopy, fourier transform infrared spectra and diffused reflectance UV–Vis spectrum and a possible growth mechanism of the ZnO nanorods was proposed. As-obtained products were utilized as photo-anode electrode in dye-sensitized solar cells and ZnO nanostructures were deposited on FTO via electrophoresis-based method. Moreover, effect of ethylenediamine and hydrazine on morphology and consequently, on solar cells efficiency was evaluated. The results showed that particle size and morphology have salient effect on solar cells efficiency and rod-like nanostructures of ZnO with smaller length and diameter have higher efficiency compared to spherical ZnO nanostructures. In addition, depositing of ZnO nanorods on ZnO nanoparticles led to obtaining 3.85 % cell efficiency that in comparison with sole nanorods (2.81 %) and sole nanoparticles (2.07 %), efficiency improvements of 37 and 86 % were respectively achieved.








Similar content being viewed by others
References
K. Hongsith, N. Hongsith, D. Wongratanaphisan, A. Gardchareon, S. Phadungdhitidhada, P. Singjai, and S. Choopun (2013). Thin Solid Films 539, 260.
B. E. Hardin, H. J. Snaith, and M. D. McGehee (2012). Nat. Photon 6, 162.
S. F. Zhang, X. D. Yang, Y. Numata, and L. Y. Han (2013). Energy Environ. Sci. 6, 1443.
B. O’Regan and M. Gratzel (1991). Nature 353, 737.
Q. F. Zhang, K. Park, J. T. Xi, D. Myers, and G. Z. Cao (2011). Adv. Energy Mater. 1, 988.
T. Kawano and H. Imai (2006). Cryst. Growth Des. 6, 1054.
J. Tamaki (2005). Sens. Lett. 3, 89.
M. Kurtz, J. Strunk, O. Hinrichsen, M. Muhler, K. Fink, B. Meyer, and C. Woll (2005). Angew. Chem. Int. Ed. 44, 2790.
C. L. Yang, J. N. Wang, W. K. Ge, L. Guo, S. H. Yang, and D. Z. Shen (2001). J. Appl. Phys. 90, 4489.
M. Izaki, K. T. Mizuno, T. Shinagawa, M. Inaba, and A. Tasaka (2006). J. Electrochem. Soc. 153, C668.
S. H. Lee, S. S. Lee, J. J. Choi, J. U. Jeon, and K. Ro (2005). Microsyst. Technol. 11, 416.
L. Vayssieres, K. Keis, S. E. Lindquist, and A. Hagfeldt (2001). J. Phys. Chem. B 105, 3350.
S. Sutthana, D. Wongratanaphisan, A. Gardchareon, S. Phadungdhitidhada, P. Ruankham, and S. Choopun (2015). Energy Procedia 79, 1021.
K. J. Hartlieb, C. L. Raston, and M. Saunders (2007). Chem. Mater. 19, 5453.
Y. L. Wang, M. Guo, M. Zhang, et al. (2010). Cryst. Eng. Comm. 12, 4024.
Q. Li, Y. Chen, L. Luo, L. Wang, Y. Yu, and L. Zhai (2013). J. Alloys Compd. 560, 156.
S. Muthukumar, C. R. Gorla, N. W. Emanetoglu, S. Liang, and Y. Lu (2001). J. Cryst. Growth 225, 197.
Y. Zhang, R. E. Russo, and S. S. Mao (2005). Appl. Phys. Lett. 87, 043106.
F. Soofivand, M. Salavati-Niasari, and F. Mohandes (2013). Mater. Lett. 98, 55.
J. Yang, J. Zheng, H. Zhai, X. Yang, L. Yang, Y. Liu, J. Lang, and M. Gao (2010). J. Alloys Compd. 489, 722.
F. Yang, W. H. Liu, X. W. Wang, J. Zheng, R. Y. Shi, H. Zhao, and H. Q. Yang (2012). ACS Appl. Mater. Interfaces 4, 3852.
A. B. Djurisic, X. Chen, Y. H. Leung, and A. M. C. Ng (2012). J. Mater. Chem. 22, 6526.
J. Rodríguez, G. Feuillet, F. Donatini, D. Onna, L. Sanchez, R. Candal, M. C. Marchi, S. A. Bilmes, and F. Chandezon (2015). Mater. Chem. Phys. 151, 378.
Z. Zhang, Y. Lv, J. Yan, D. Hui, J. Yun, C. Zhai, and W. Zhao (2015). J. Alloys Compd. 650, 374.
Z. Zarghami, M. Ramezani, and M. Maddahfar (2015). Mater. Lett. 152, 21.
M. Mousavi-Kamazani and M. Salavati-Niasari (2014). Compos. Part B Eng. 56, 490.
S. Liu, Y. Cai, X. Cai, H. Li, F. Zhang, Q. Mu, Y. Liu, and Y. Wang (2013). Appl. Catal. A Gen. 453, 45.
Q. Mu and Y. Wang (2011). J. Alloys Compd. 509, 2060.
F. Mohandes and M. Salavati-Niasari (2013). Ultrason. Sonochem. 20, 354.
N. Salehifar, Z. Zarghami, and M. Ramezani (2016). Mater. Lett. 167, 226.
Z. Zarghami, M. Maddahfar, and M. Ramezani (2015). J. Mater. Sci. Mater. Electron. 26, 6339.
J. Fang, H. Fan, H. Tian, and G. Dong (2015). Mater. Charact. 108, 51.
Acknowledgments
This work was supported by the Chemistry Research Center at Islamic Azad University, Arak.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zarghami, Z., Ramezani, M. & Motevalli, K. Zno Nanorods/Nanoparticles: Novel Hydrothermal Synthesis, Characterization and Formation Mechanism for Increasing the Efficiency of Dye-Sensitized Solar Cells. J Clust Sci 27, 1451–1462 (2016). https://doi.org/10.1007/s10876-016-1011-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-016-1011-1