Abstract
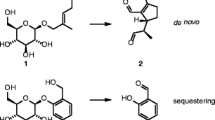
Beetles share with other eukaryotes an innate immune system that mediates endogenous defense against pathogens. In addition, larvae of some taxa produce fluid exocrine secretions that contain antimicrobial compounds. In this paper, we provide evidence that larvae of the brassy willow leaf beetle Phratora vitellinae constitutively release volatile glandular secretions that combat pathogens in their microenvironment. We identified salicylaldehyde as the major component of their enveloping perfume cloud, which is emitted by furrow-shaped openings of larval glandular reservoirs and which inhibits in vitro the growth of the bacterial entomopathogen Bacillus thuringiensis. The suggested role of salicylaldehyde as a fumigant in exogenous antimicrobial defense was confirmed in vivo by its removal from glandular reservoirs. This resulted in an enhanced susceptibility of the larvae to infection with the fungal entomopathogens Beauveria bassiana and Metarhizium anisopliae. Consequently, we established the hypothesis that antimicrobial defense in beetles can be expanded beyond innate immunity to include external disinfection of their microenvironment, and we report for the first time the contribution of fumigants to antimicrobial defense in animals.





Similar content being viewed by others
References
Altincicek B., Knorr E., and Vilcinskas A. 2007. Beetle immunity: Identification of immune-inducible genes from the model insect Tribolium castaneum. Dev. Comp. Immunol., DOI 10.1016/j.dci.2007.09.005.
Bärlocher, F. 1999. Biostatistik. Thieme Publ., Stuttgart, Germany.
Blum, M. S., Brand, J. M., Wallace, J. B., and Fales, H. M. 1972. Chemical characterisation of the defensive secretion of a chrysomelid larva. Life Sci. 11:525–531.
Clarkson, J. M., and Charnley, A. K. 1996. New insights into the mechanisms of fungal pathogenesis in insects. Trends Microbiol. 4:197–203.
Dettner, K. 1985. Ecological and phylogenetic significance of defensive compounds from pygidial glands of Hydradephaga (Coleoptera). Proc. Acad. Nat. Sci. Philadelphia 137:156–171.
Dettner, K., Fettköther, R., Ansteeg, O., Deml, R., Liepert, C., Petersen, B., Haslinger, E., and Francke, W. 1992. Insecticidal fumigants from defensive glands of insects—a fumigant test with adults of Drosophila melanogaster. J. Appl. Entomol. 113:128–137.
Freitak, D., Wheat, C. W., Heckel, D. G., and Vogel, H. 2007. Immune system responses and fitness costs associated with consumption of bacteria in larvae of Trichoplusia ni. BMC Biology, DOI 10.1186/1741-7007-5-56.
Garb, G. 1915. The eversible glands of a chrysomelid larva, Melasoma lapponica. J. Entomol. Zool. 7:87–97.
Gillespie, J. P., Bailey, A. M., Cobb, B., and Vilcinskas, A. 2000. Fungi as elicitors of insect immune responses. Arch. Insect Biochem. Physiol. 44:49–68.
Grégoire, J.-C. 1988. Larval gregariousness in the Chrysomelidae, pp. 253–260, in P. H., Jolivet, E., Petitpierre, T. H., and Hsiao (eds.). Biology of ChrysomelidaeKluwer Academic Publisher, Dordrecht, The Netherlands.
Gross, J., and Hilker, M. 1995. Chemoecological studies of the exocrine glandular larval secretions of two chrysomelid species (Coleoptera): Phaedon cochleariae and Chrysomela lapponica. Chemoecology 5/6:185–189.
Gross, J., Müller, C., Vilcinskas, A., and Hilker, M. 1998. Antimicrobial activity of the exocrine glandular secretions, hemolymph and larval regurgitate of the mustard leaf beetle Phaedon cochleariae. J. Invertebr. Pathol. 72:296–303.
Gross, J., Podsiadlowski, L., and Hilker, M. 2002. Antimicrobial activity of exocrine glandular secretion of Chrysomela larvae. J. Chem. Ecol. 28:317–331.
Gross, J., Fatouros, N. E., Neuvonen, S., and Hilker, M. 2004. The importance of specialist natural enemies for Chrysomela lapponica in pioneering a new hostplant. Ecol. Entomol. 29:584–593.
Herzner, G., and Strohm, E. 2007. Fighting fungi with physics: Food wrapping by a solitary wasp prevents water condensation. Curr. Biol. 17:R46–R47.
Herzner, G., Schmitt, T., Peschke, K., Hilpert, A., and Strohm, E. 2007. Food wrapping with the postpharyngeal gland secretion by females of the European beewolf Philanthus triangulum. J. Chem. Ecol. 33:849–859.
Hilker, M., and Schulz, S. 1994. Composition of larval secretion of Chrysomela lapponica (Coleoptera, Chrysomelidae) and its dependence on host plant. J. Chem. Ecol. 20:1075–1093.
Hinton, H. E. 1951. On a little-known protective device of some chrysomelid pupae (Coleoptera). Proc. R. Entomol. Soc. Lond. 26:67–73.
Hoffmann, J. A. 2003. The immune response of Drosophila. Nature 426:33–38.
Holm, S. 1979. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 6:65–70.
Humber, R. A. 1996. Fungal pathogens of the Chrysomelidae and prospects of their use in biological control, pp. 93–115, in P. H., Jolivet, M. L., and Cox (eds.). Chrysomelidae Biology, SPB Academic Publ., Amsterdam, The Netherlands.
Köpf, A., Rank, N. E., Roininen, H., and Tahvanainen, J. 1997. Defensive larval secretions of leaf beetles attract a specialist predator Parasyrphus nigritarsis. Ecol. Entomol. 22:176–183.
Kovac, D., and Maschwitz, U. 1990. Secretion-grooming in aquatic beetles (Hydradephaga): A chemical protection against contamination of the hydrofuge respiratory region. Chemoecology 1:131–138.
Kuhn, J., Pettersson, E. M., Feld, B., Burse, A., Termonia, A., Pasteels, J. M., and Boland, W. 2004. Selective transport systems mediate sequestration of plant glucosides in leaf beetles: A molecular basis for adaptation and evolution. Proc. Natl. Acad. Sci. U. S. A. 101:13808–13813.
Nahrung, H. F., Dunstan, P. K., and Allen, G. R. 2001. Larval gregariousness and neonate establishment of the eucalypt-feeding beetle Chrysophtharta agricola (Coleoptera: Chrysomelidae: Paropsini). Oikos 94:358–364.
Oldham, N. J., Veith, M., and Boland, W. 1996. Iridoid monoterpene biosynthesis in insects: evidence for a de novo pathway occurring in the defensive glands of Phaedon armoraciae (Chrysomelidae) leaf beetle larvae. Naturwissenschaften 83:470–473.
Pasteels, J. M., Braekman, J.-C., Daloze, D., and Ottinger, R. 1982. Chemical defence in chrysomelid larvae and adults. Tetrahedron 38:1891–1897.
Pasteels, J. M., Daloze, D., and Rowell-Rahier, M. 1986. Chemical defence in chrysomelid eggs and neonate larvae. Physiol. Entomol. 11:29–37.
Pasteels, J. M., Braekman, J.-C., and Daloze, D. 1988. Chemical defence in the Chrysomelidae, pp. 233–252, in P. H. Jolivet, E. Petitpierre, and T. H. Hsiao (eds.). Biology of ChrysomelidaeKluwer Academic Publ., Dordrecht, The Netherlands.
Rosengaus, R. B., Lefebvre, M. L., and Traniello, J. F. A. 2000. Inhibition of fungal spore germination by Nasutitermes: Evidence for a possible antiseptic role of soldier defensive secretions. J. Chem. Ecol. 26:21–39.
Rostás, M., and Hilker, M. 2002. Feeding damage by larvae of the mustard leaf beetle deters conspecific females from oviposition and feeding. Entomol. Exp. Appl. 103:267–277.
Rowell-Rahier, M., and Pasteels, J. M. 1986. Economics of chemical defense in Chrysomelinae. J. Chem. Ecol. 12:1189–1203.
Royet, J., Reichhart, J. M., and Hoffmann, J. A. 2005. Sensing and signaling during infection in Drosophila. Curr. Opin. Immunol. 17:11–17.
StatSoft I. 1999. STATISTICA for Windows users manual, version 5.5.
Traniello, J. F., Rosengaus, R. B., and Savoie, K. 2002. The development of immunity in a social insect: Evidence for the group facilitation of disease resistance. Proc. Natl. Acad. Sci. U. S. A. 99:6838–6842.
Tribolium Genome Sequencing Consortium 2008. The genome of the developmental model beetle and pest Tribolium castaneum. Nature: in press
Vilcinskas, A., and Götz, P. 1999. Parasitic fungi and their interactions with the insect immune system. Adv. Parasitol. 43:268–313.
Vilcinskas, A., and Gross, J. 2005. Drugs from bugs: The use of insects as a valuable source of transgenes with potential in modern plant protection strategies. Journal of Pest Science 78:187–191.
Wain, R. L. 1943. The secretion of salicylaldehyde by the larvae of the brassy willow beetle (Phyllodecta vitellinae L.). Ann. Rep. Agric. Horticult. Res. Stat. 108–110.
Wallace, J. B., and Blum, M. S. 1969. Refined defensive mechanisms in Chrysomela scripta. Ann. Entomol. Soc. Am. 62:503–506.
Wilson, K., Knell, R., Boots, M., and Koch-Osborne, J. 2003. Group living and investment in immune defence: an interspecific analysis. J. Anim. Ecol. 72:133–143.
Zvereva, E. L., and Rank, N. E. 2004. Fly parasitoid Megaselia opacicornis uses defensive secretions of the leaf beetle Chrysomela lapponica to locate its host. Oecologia 140:516–522.
Acknowledgments
We thank Gisbert Zimmermann (BBA Darmstadt, Germany) for providing the different strains of entomopathogenic bacteria and fungi and Monika Hilker (Berlin, Germany) for providing the GC-MS for analysis of headspace samples. The authors are indebted to Rod Snowdon (Giessen, Germany) for editing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gross, J., Schumacher, K., Schmidtberg, H. et al. Protected by Fumigants: Beetle Perfumes in Antimicrobial Defense. J Chem Ecol 34, 179–188 (2008). https://doi.org/10.1007/s10886-007-9416-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-007-9416-9