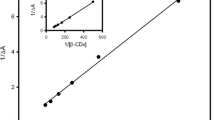
Abstract
The photophysical behaviour of 2,6-diaminopyridine (DAP) has been studied in solvents of different polarity, pH, β-cyclodextrin (β-CD) and compared with 2-amino pyridine (2AP). The inclusion complex of both molecules with β-CD are analysed by UV-visible, fluorimetry, FT-IR, 1H NMR, SEM and AM1 methods. The solvent studies shows i) DAP gives more red shifted absorption and emission maxima than 2AP molecule and ii) addition of amino group in 2AP effectively increase the resonance interaction in the pyridine ring. A regular red shift observed in acidic pH solutions suggests intramolecular proton transfer (IPT) present in both molecules. β-CD studies indicates i) in pH ~ 7, a regular red shifted absorption and emission maxima observed in AP molecules suggests pyridine ring encapsulated in to the β-CD cavity (1:1 inclusion complex formed) and ii) in pH ~ 1, a blue shifted absorption maxima noticed in 2AP, is due to protonated amino group deeply encapsulated in to the hydrophobic part of the β-CD cavity.










Similar content being viewed by others
References
Schulman SG (1974) In: Kortitzky AR (ed) Physical methods in heterocyclic chemistry, vol VI. Academic, New York, p 193
Barni P, Savarino P, Viscardi G (1991) Trends Heterocycl Chem 2:27
Brand L, Gohlka JR (1972) Annu Rev Biochem 41:843
Klose G, Stelzmer F (1974) Biochem Biophys Acta 1:363
Smets J, Maes G (1991) Chem Phys Lett 187:532
Brown RS, Tse A, Vaderas JC (1980) J Am Chem Soc 102:1174
Kwiatkowski JS, Bartlett RJ, Person WB (1988) J Am Chem Soc 110:2353
King ST, Oilling DL, Teferkiller NB (1972) Tetrahedron 28:5859
Cox RH, Bothner AA (1969) J Phys Chem 73:2465
Krebs C, Hofmann H, Kohler HJ, Weiss C (1980) Chem Phys Lett 69:537
Mitra S, Das R, Mukherjee S (1994) Spectrochim Acta 50A:1301
Mitra S, Das R, Mukherjee S (1994) J Photochem Photobiol 79:49
Mitra S, Das R, Mukherjee S (1999) J Lumin 81:61
Will G, Mukherjee S et al (1999) Indian J Chem 38A:753
Jorgenson MJ, Hartter DA (1963) J Am Chem Soc 85:878
Yagil G (1967) J Phys Chem 71:1054
Marckwald G (1894) Berlin J 27:1321
EA Steck, Ewing GW (1948) J Am Chem Soc 3397
Maier-Bode H, Altpeter J (1934) Das Pyridin und seine derivate Knapp, Halle, p 91
Tway PC, Love LJC (1982) J Phys Chem 86:5223
Dey JK, Warner IM (1998) J Photochem Photobiol A: Chem 116:27
Biswas A, Dey JK (2001) Indian J Chem 40A:1143
Lim EC (1977) In: Lim EC (ed) Excited states, vol 3. Academic, New York, p 305
Hochstrasser RM, Merzzacco CA (1969) In: Lim EC (ed) Molecular luminescence. W.A. Berjamin Inc, New York, p 631
Lippert E (1955) Z Naturforsch 10A:541
Biolet L, Kawski A (1962) Z Naturforsch 17A:621
Reichardt C, Dimroth K (1968) Forstschr Chem Forsch 11:1
Rajendiran N, Swaminathan M (1995) J Photochem Photobiol A: Chem 90:109
Rajendiran N, Swaminathan M (1996) Bull Chem Soc Jpn 69:2447
Rajendiran N, Swaminathan M (1996) Indian J Chem 35A:818
Rajendiran N, Swaminathan M (1995) Bull Chem Soc Jpn 68:2802
Rajendiran N, Swaminathan M (1996) J Photochem Photobiol A: Chem 93:103
Stalin T, Rajendiran N (2007) Spectrochim Acta 68A:894
Stalin T, Rajendiran N (2008) Spectrochim Acta 69A:822
Stalin T, Rajendiran N (2006) J Mol Struct 795:35
Stalin T, Rajendiran N (2006) Chem Phys 322:311
Stalin T, Rajendiran N (2006) J Photochem Photobiol A: Chem 18:137
Stalin T, Rajendiran N (2006) J Incl Phenom Macrocycl Chem 55:21
Krishnamoorthy G, Dogra SK (1999) J Photochem Photobiol A: Chem 123:109
Das S (2002) Chem Phys Lett 361:21
Kim TH, Cho DW, Yoon M, Kim D (1996) J Phys Chem 160:15670
Kim TH, Cho DW, Yoon M, Kim D (1996) J Chem Soc Faraday Trans 92:29
Jiang TB (1995) J Photochem Photobiol A: Chem 88:109
Agbaria RA, Uzar B, Gill D (1989) J Phys Chem 93:3855
Benesi HA, Hildebrand JH (1949) J Am Chem Soc 71:2703
Senger M (1984) In: Atwood JL, Davies JED, Macnicol DD (eds) Inclusion complexes, vol 2. Academic, London, p 231
Lehman J, Klienpeter E (1991) J Incl Phenom 10:233
Inoue Y, Kuan F, Chujo R (1987) Bull Chem Soc Jpn 60:253
Chao JB, Tong HB, Huang SP, Lie DS (2004) Spectrochim Acta 60A:161
Acknowledgement
This work is supported by the Department of Science and Technology, New Delhi, (Fast Track Proposal–Young Scientist Scheme No. SR/FTP/CS-14/2005) and University Grants Commission, New Delhi (Project No. F-31-98/2005 (SR).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Prabhu, A.A.M., Siva, S., Sankaranarayanan, R.K. et al. Intramolecular Proton Transfer Effects on 2,6-diaminopyridine. J Fluoresc 20, 43–54 (2010). https://doi.org/10.1007/s10895-009-0520-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-009-0520-9