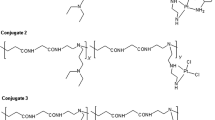
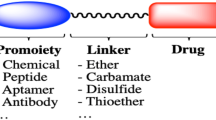
Abstract
Prodrugs optimize the absorption, distribution, metabolism, excretion and reduce unwanted toxicity of the parent drugs. The medicinal application of polymeric prodrugs for delivery of metal-based anticancer drugs is a field of increasing prominence because metal-based drugs offer possibilities for the design of therapeutic agents that are not readily available to organic compounds. This review is focused on the progress of polymeric prodrugs of metal based anticancer agents. A better understanding of the pharmacokinetics of these prodrugs will provide a rational for their further development into anticancer drugs for overcoming multidrug resistance.








Similar content being viewed by others
References
T. Finkel, M. Serrano, M.A. Blasco, The common biology of cancer and ageing. Nature 448, 767–774 (2007)
A.F. Chambers, A.C. Groom, I.C. MacDonald, Nature Rev. Cancer 2, 563 (2002)
WHO fact sheet, No 297, February 2014. http://www.who.int/mediacentre/factsheets/fs297/en/. Accessed 4th October 2014
C. de Martel, J. Ferlay, S. Franceschi, J. Vignat, F. Bray, D. Forman, M. Plummer, Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 13, 607–615 (2012)
E.W. Neuse, Synthetic polymers as drug-delivery vehicles in medicine. Metal-Based Drugs 2008, 469531 (2008). doi:10.1155/2008/469531. Hindawi Publishing Corporation
A.Y. Shaikh, J.A. Shih, Chemotherapy-induced cardiotoxicity. Curr. Heart Fail Rep 9, 117–127 (2012)
A.V. Thatishetty, N. Agresti, C.B. O’Brien, Chemotherapy-induced hepatotoxicity. Clin. Liver Dis. 17, 671–686 (2013)
B.D. Humphreys, R.J. Soiffer, C.C. Magee, Renal failure associated with cancer and its treatment: an update. J. Am. Soc. Nephrol. 16, 151–161 (2005)
E. Crowley, C.A. McDevitt, R. Callaghan, Multidrug Resistance in Cancer. Generating Inhibitors of P-Glycoprotein: Where to, Now? (Humana Press, New York, 2009), pp. 405–432. Springer Protocols
M. Hacker, W.S. Messer II, K.A. Bachmann, Pharmacology: Principles and Practice (Academic Press, Waltham, 2009), pp. 216–217
K.M. Huttunen, H. Raunio, J. Rautio, Prodrugs from serendipity to rational design. Pharmacol. Rev. 63, 750–771 (2011)
K.-M. Wu, A new classification of prodrugs: regulatory perspectives. Pharmaceuticals 2, 77–81 (2009)
H. Maeda, SMANCS and polymer-conjugated macromolecular drugs: advantages in cancer chemotherapy. Adv. Drug Deliv. Rev. 46, 169–185 (2001)
Y. Matsumura, H. Maeda, A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 46, 6387–6392 (1986)
N.A. Rohini, A. Joseph, A. Mukerji, Polymeric prodrugs: recent achievements and general strategies. J. Antivir. Antiretrovir 2, S15 (2013)
S. Maher, D. Toomey, C. Condron, D. Bouchier-Hayes, Activation-induced cell death: the controversial role of Fas and Fas ligand in immune privilege and tumour counterattack. Immunol. Cell Biol. 80, 131–137 (2002)
C.M. Peterson, J.M. Lu, Y. Sun, C.A. Peterson, J.G. Shiah, R.C. Straight, J. Kopeček, Combination chemotherapy and photodynamic therapy with N-(2-hydroxypropyl) methacrylamide copolymer-bound anticancer drugs inhibit human ovarian carcinoma. Cancer Res. 56, 3980–3985 (1996)
G. Pasut, F.M. Veronese, Polymer–drug conjugation, recent achievements and general strategies. Prog. Polym. Sci. 32, 933–961 (2007)
Y. Fang, G. Zheng, J. Yang, H. Tang, Y. Zhang, B. Kong, Y. Lv, C. Xu, A.M. Asiri, J. Zi, F. Zhang, D. Zhao, Dual-pore mesoporous carbon@silica composite core-shell nanospheres for multidrug delivery. Angew. Chem. 126, 5470–5474 (2014)
M. Vallet-Regi, Nanostructured mesoporous silica matrices in nanomedicine. J. Intern. Med. 267, 22–43 (2010)
A.M. El-Toni, M.A. Ibrahim, J.P. Labis, A. Khan, M. Alhoshan, Optimization of synthesis parameters for mesoporous shell formation on magnetic nanocores and their application as nanocarriers for docetaxel cancer drug. Int. J. Mol. Sci. 14, 11496–11509 (2013)
N.J. Wheate, D.P. Buck, A.I. Day, J.G. Collins, Cucurbit[n]uril binding of platinum anticancer complexes. Dalton Trans. 3, 451–458 (2006)
N.J. Wheate, Improving platinum (II)-based anticancer drug delivery using cucurbit[n]urils. J. Inorg. Biochem. 102, 2060–2066 (2008)
A.R. Kennedy, A.J. Florence, F.J. McInnes, N.J. Wheate, A chemical preformulation study of a host–guest complex of cucurbit[7]uril and a multinuclear platinum agent for enhanced anticancer drug delivery. Dalton Trans. 37(2009), 7695–7700 (2009)
Z. Mahdavifar, S. Samiee, Theoretical investigation of inclusion complex formation of Gold(III)—Dimethyldithiocarbamate anticancer agents with cucurbit[n = 5,6]urils. Arabian J. Chem. 7, 425–435 (2014)
M.P.M. Marques, Platinum and palladium polyamine complexes as anticancer agents: the structural factor. ISRN Spectrosc. 2013, 287353 (2013). Hindawi Publishing Corporation
H. Ringsdorf, Structure and properties of pharmacologically active polymers. J. Polym. Sci.: Pol. Sym. 51, 135–153 (1975)
S. Jaracz, J. Chen, L.V. Kuznetsova, I. Ojima, Recent advances in tumor-targeting anticancer drug conjugates. Bioorg. Med. Chem. 13, 5043–5054 (2005)
N. Larson, H. Ghandehari, Polymeric conjugates for drug delivery. Chem. Mater. 24, 840–853 (2012)
Dendrimers. http://en.wikipedia.org/wiki/Dendrimers. Accessed 12th October 2014
S. Svenson, D.A. Tomalia, Dendrimers in biomedical applications-reflections on the field. Adv. Drug Deliv. Rev. 57, 2106–2129 (2005)
N. Vijayalakshmi, A. Ray, A. Malugin, H. Ghandehari, Carboxyl-terminated PAMAM-SN38 conjugates: synthesis characterization, and in vitro evaluation. Bioconjugate Chem. 21, 1804–1810 (2010)
I.J. Majoros, C.R. Williams, A. Becker, J.R. Baker Jr, Methotrexate delivery via folate targeted dendrimer-based nanotherapeutic platform Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 1, 502–510 (2009)
D. Bhadra, S. Bhadra, S. Jain, N.K. Jain, A PEGylated dendritic nanoparticulate carrier of fluorouracil. Int. J. Pharm. 257, 111–124 (2003)
H. He, Y. Li, X.R. Jia, J. Du, X. Ying, W.L. Lu, J.N. Lou, Y. Wei, PEGylated Poly(amidoamine) dendrimer-based dual-targeting carrier for treating brain tumors. Biomaterials 32, 478–487 (2011)
Y. Cheng, Z. Xu, M. Ma, T.J. Xu, Dendrimers as drug carriers: applications in different routes of drug administration. Pharm. Sci. 97, 123–143 (2008)
R. Wiwattanapatapee, B. Carreno-Gomez, N. Malik, R. Duncan, Anionic PAMAM dendrimers rapidly cross adult rat intestine in vitro: a potential oral delivery system? Pharm. Res. 17, 991–998 (2000)
A.R. Menjoge, A.L. Rinderknecht, R.S. Navath, M. Faridnia, C.J. Kim, R. Romero, R.K. Miller, R.M.J. Kannan, Transfer of PAMAM dendrimers across human placenta: prospects of its use as drug carrier during pregnancy. Controll. Release 150, 326–338 (2011)
M.T. Morgan, Y. Nakanishi, D.J. Kroll, A.P. Griset, M.A. Carnahan, M. Wathier, N.H. Oberlies, G. Manikumar, M.C. Wani, M.W. Grinstaff, Dendrimer-encapsulated camptothecins: increased solubility, cellular uptake, and cellular retention affords enhanced anticancer activity in vitro. Cancer Res. 66, 11913–11921 (2006)
R.K. Tekade, T. Dutta, V. Gajbhiye, N.K. Jain, Exploring dendrimer towards dual drug delivery. J. Microencapsul. 26, 287–296 (2009)
G. Vilar, J. Tulla-Puchea, F. Albericio, Polymers and drug delivery systems. Current Drug Deliv. 9, 367–394 (2012)
T.R. Hoare, D.S. Kohane, Hydrogels in drug delivery: progress and challenges. Polymer 49, 1993–2007 (2008)
G. Riess, Micellization of block copolymers. Prog. Polym. Sci. 28, 1107–1170 (2003)
S.E. Dunn, A. Brindley, S.S. Davis, M.C. Davies, L. Illum, Polystyrene–poly(ethylene glycol) (PS-PEG2000) particles as model systems for site specific drug delivery. 2. The effect of PEG surface density on the in vitro cell interaction and in vivo biodistribution. Pharm. Res. 11, 1016–1022 (1994)
R.K. Jain, Delivery of molecular and cellular medicine to solid tumors. Adv. Drug Deliv. Rev. 26, 71–90 (1997)
W. Xu, P. Ling, T. Zhang, Polymeric micelles, a promising drug delivery system to enhance bioavailability of poorly water-soluble drugs. J. Drug Deliv. 2013, 340315 (2013)
B. Kastenholz, Important contributions of a new quantitative preparative native continuous polyacrylamide gel electrophoresis (QPNC-PAGE) procedure for elucidating metal cofactor metabolisms in protein-misfolding diseasesa theory. Electroanalysis 18, 103–106 (2006)
W.M. Kwiatek, T. Drewniak, M. Gajda, M. Galka, A.L. Hanson, T. Cichocki, Preliminary study on the distribution of selected elements in cancerous and non-cancerous kidney tissues. J. Trace Elem. Med Biol. 16, 155–160 (2002)
S.K. Bharti, S.K. Singh, Recent developments in the field of anticancer metallopharmaceuticals. Int. J. Pharm Tech Res. 1, 1406–1420 (2009)
M.J. Clarke, Ruthenium metallopharmaceuticals. Coord. Chem. Rev. 236, 209–233 (2003)
V. Brabec, O. Nov´akov´a, DNA binding mode of ruthenium complexes and relationship to tumor cell toxicity. Drug Resist. Updates 9, 111–122 (2006)
C.G. Hartinger, M.A. Jakupec, S. Zorbas-Seifrieda, M. Groessl, A. Egger, W. Berger, H. Zorbas, P.J. Dyson, B.K. Keppler, KP1019, a new redox-active anticancer agent-Preclinical development and results of a clinical phase I study in tumor patients. Chem. Biodivers. 5, 2140–2155 (2008)
E. Alessio, G. Mestroni, A. Bergamo, G. Sava, E. Alessio, G. Mestroni, A. Bergamo, G. Sava, Ruthenium antimetastatic agents. Curr. Topics Med. Chem. 4, 1525–1535 (2004)
J. Rodrigues, M.G. Jardim, J. Figueira, M. Gouveia, H. Tomàs, K. Rissanen, Poly(alkylidenamines) dendrimers as scaffolds for the preparation of low-generation ruthenium based metallodendrimers. New J. Chem. 35, 1938–1943 (2011)
P. Govender, A.K. Renfrew, C.M. Clavel, P.J. Dyson, B. Therrien, G.S. Smith, Antiproliferative activity of chelating N, O- and N, N-ruthenium(II) arene functionalised poly(propyleneimine) dendrimer scaffolds. Dalton Trans. 40, 1158–1167 (2011)
P. Govender, L.C. Sudding, C.M. Clavel, P.J. Dyson, B. Therrien, S. Smith, The influence of RAPTA moieties on the antiproliferative activity of peripheral-functionalised poly(salicylaldiminato) metallodendrimers. Dalton Trans. 42, 1267–1277 (2013)
A. Valente, M.H. Garcia, F. Marques, Y. Miao, C. Rousseau, P. Zinck, First polymer “ruthenium-cyclopentadienyl” complex as potential anticancer agent. J. Inorg. Biochem. 127, 79–81 (2013)
P. Heffeter, A. Riabtseva, Y. Senkiv, C.R. Kowol, W. Körner, U. Jungwith, N. Mitina, B.K. Keppler, T. Konstantinova, I. Yanchuk, R. Stoika, A. Zaichenko, W. Berger, Nanoformulation improves activity of the (pre)clinical anticancer ruthenium complex KP1019. J. Biomed. Nanotechnol. 10, 877–884 (2014)
A. Pitto-Barry, N.P.E. Barry, O. Zava, R. Deschenaux, B. Therrien, Encapsulation of pyrene-functionalized poly(benzyl ether) dendrons into a water-soluble organometallic cage. Chem. Asian J. 6, 1595–1603 (2011)
B.M. Blunden, D.S. Thomas, M.H. Stenzel, Macromolecular ruthenium complexes as anti-cancer agents. Polym. Chem. 3, 2964 (2012)
P. Umapathy, The chemical and biochemical consequences of the binding of the antitumour drug cisplatin and other platinum group metal complexes to DNA. Coordin. Chem. Rev. 95, 129–181 (1989)
M.P.M. Marques, Platinum and palladium polyamine complexes as anticancer agents: the structural factor. ISRN Spectrosc. 2013, 287353 (2013). doi:10.1155/2013/287353. Hindawi Publishing Corporation
M. Tanaka, H. Kataoka, S. Yano, H. Ohi, K. Kawamoto, T. Shibahara, T. Mizoshita, Y. Mori, S. Tanida, T. Kamiya, T. Joh, Anti-cancer effects of newly developed chemotherapeutic agent, glycoconjugated palladium (II) complex, against cisplatin-resistant gastric cancer cells. BMC Cancer 13, 237 (2013)
T.M. Silva, S.M. Fiuza, M.P.M. Marques, L. Persson, S. Oredsson, Increased breast cancer cell toxicity by palladination of the polyamine analogue N1, N11-bis(ethyl)norspermine. Amino Acids 46, 339–352 (2014)
B. Ghalandari, A. Divsalar, A.A. Saboury, K. Parivar, The new insight into oral drug delivery system based on metal drugs in colon cancer therapy through β lactoglobulin/oxali-palladium nanocapsules. J. Photochem. Photobiol. B: Biol. 140, 255–265 (2014)
B. Ghalandari, A. Divsalar, A.A. Saboury, T. Haertlé, K. Parivar, R. Bazl, M. Eslami-Moghadam, M. Amanlou, Spectroscopic and theoretical investigation of oxali-palladium interactions with β-lactoglobulin. Spectrochim. Acta A Mol. Biomol. Spectrosc. 24, 1038–1046 (2014)
E.R. Tiekink, Gold derivatives for cancer treatment. Bioinorg. Chem. Applns. 53, 1–9 (2003)
I. Kostova, Gold coordination complexes as anticancer agents. Anticancer Agents Med. Chem. 6, 19–32 (2006)
L. Ronconi, L. Giovagnini, C. Marzano, F. Bettio, R. Graziani, G. Pilloni, D. Fregona, Gold dithiocarbamate derivatives as potential antineoplastic agents: design, spectroscopic properties and in vitro antitumor activity. Inorg. Chem. 44, 1867–1881 (2005)
L. Ronconi, C. Marzano, P. Zanello, M. Corsini, G. Miolo, C. Macca, A. Trevisan, D. Fregona, Gold(III) dithiocarbamate derivatives for the treatment of cancer: solution chemistry, DNA binding, and hemolytic properties. J. Med. Chem. 49, 1648–1657 (2006)
C.M. Che, R.W.Y. Sun, W.Y. Yu, C.B. Ko, N. Zhu, H. Sun, Gold(III) porphyrins as a new class of anticancer drugs: cytotoxicity, DNA binding and induction of apoptosis in human cervix epitheloid cancer cells. Chem. Commun. 14, 1718–1719 (2003)
C.T. Lum, L. Huo, R.W.Y. Sun, M. Li, H.F. Kung, C.M. Che, M.C. Lin, Gold(III) porphyrin 1a prolongs the survival of melanoma-bearing mice and inhibits angiogenesis. Acta Oncol. 50, 719–726 (2011)
Y.F. To, R.W.Y. Sun, Y. Chen, V.S. Chan, W.Y. Yu, P.K.H. Tam, C.M. Che, M.C.L.S. Lin, Gold(III) porphyrin complex is more potent than cisplatin in inhibiting growth of nasopharyngeal carcinoma in vitro and in vivo. Int. J. Cancer 124, 1971–1979 (2009)
C.T. Lum, X. Liu, R.W.Y. Sun, X.P. Li, Y. Peng, H.F. Kung, C.M. Che, M.C. Lin, Gold(III) porphyrin 1a inhibited nasopharyngeal carcinoma metastasis in vivo and inhibited cell migration and invasion in vitro. Cancer Lett. 294, 159–166 (2010)
C.T. Lum, A.S.T. Wong, M.C.M. Lin, C.M. Che, R.W.Y. Sun, A gold(III) porphyrin complex as an anti-cancer candidate to inhibit growth of cancer-stem cells. Chem. Commun. 49, 4364–4366 (2012)
J.J. Yan, R.W.Y. Sun, P. Wu, M.C.M. Lin, A.S.C. Chan, C.-M. Che, Encapsulation of dual cytotoxic and anti-angiogenic gold(III) complexes by gelatin-acacia microcapsules: In vitro and in vivo studies. Dalton Trans. 39, 7700–7705 (2010)
P. Lee, R. Zhang, V. Li, X. Liu, R.W. Sun, C.M. Che, K.K. Wong, Enhancement of anticancer efficacy using modified lipophilic nanoparticle drug encapsulation. Int. J. Nanomed. 7, 731–737 (2012)
J.J. Zhang, W. Lu, R.W.Y. Sun, C.M. Che, Organogold(III) supramolecular polymers for anticancer treatment. Angew. Chem. Int. Ed. 51, 4882–4886 (2012)
C. Marzano, M. Pellei, F. Tisato, C. Santini, Copper Complexes as Anticancer Agents. Anti-Cancer Agents Med. Chem. 9, 185–211 (2009)
D.S. Sigman, A. Mazumder, D.M. Perrin, Chemical nucleases. Chem. Rev. 93, 2295–2316 (1993)
A. Pramanik, D. Laha, P. Pramanik, P. Karmakar, A novel drug “copper acetylacetonate” loaded in folic acid-tagged chitosan nanoparticle for efficient cancer cell targeting. J. Drug Target. 22, 23–33 (2014)
R.S. Kumar, S. Arunachalam, V.S. Periasamy, C.P. Preethy, A. Riyasdeen, M.A. Akbarsha, DNA binding and biological studies of some novel water-soluble polymer–copper(II)–phenanthroline complexes. Eur. J. Med. Chem. 43, 2082–2091 (2008)
R.S. Kumar, V.S. Periasamy, C.P. Paul, A. Riyasdeen, S. Arunachalam, M.A. Akbarsha, Cytotoxic effect of a polymer–copper(II) complex containing2,2-bipyridyl ligand on human lung cancer cells. Med. Chem. Res. 20, 726–731 (2011)
S. Ambika, S. Arunachalam, R. Arunb, K. Premkumar, Synthesis, nucleic acid binding, anticancer and antimicrobial activities of polymer–copper(II) complexes containing intercalative phenanthroline ligand(DPQ). RSC Adv. 3, 16456–16468 (2013)
M. Gielen, H. Ma, A. Bouhdid, H. Dalil, M. Biesemans, R. Willem, Di-n-butyl-tri-n- butyl- and triphenyltin dl-terebates: synthesis characterization and in vitro antitumour activity. Met.-Based Drugs 4, 193–197 (1997)
Q. Li, F.M.C. Guedes da Silva, A.J.L. Pombeiro, Diorganotin(iv) derivatives of substituted benzohydroxamic acids with high antitumor activity. Chemistry: A European Journal 10, 1456–1462 (2004). doi:10.1002/chem.200305266
L. Yunlan, L. Jinjie, L. Qingshan, Mechanisms by which the antitumor compound di-n-butyl-di-(4-Chlorobenzohydroxamato)tin(IV)induces apoptosis and the mitochondrial-mediated signaling pathway in human cancer sgc-7901 cells. Mol. Carcinog. 49, 566–581 (2010)
S. Tabassum, C. Pettinari, Organotin(IV) derivatives of l-cysteine and their in vitro anti-tumor properties. J. Organomet. Chem. 691, 1761–1766 (2006)
T. Li, L. Yunlan, G. Rui, Q.S. Li, Oxidative stress in di-n-butyl-di-(4-chloro- benzohydroxamato)tin (IV)-induced hepatotoxicity determined by proteomic profiles. Toxicol. Lett. 213, 167–173 (2012)
K. Navakoski de Oliveira, V. Andermark, S. von Grafenstein, L.A. Onambele, G. Dahl, R. Rubbiani, G. Wolber, C. Gabbiani, L. Messori, A. Prokop, I. Ott, Butyltin(IV) benzoates: inhibition of thioredoxin reductase, tumor cell growth inhibition, and interactions with proteins. ChemMedChem 8, 256–264 (2013)
X. Shang, J. Cui, J. Wu, A.J.L. Pombeiro, Q. Li, Polynuclear diorganotin(IV) complexes with arylhydroxamates: syntheses, structures and in vitro cytotoxic activities. J. Inorg. Biochem. 102, 901–909 (2008)
C.E. Carraher Jr, T.S. Sabir, M.R. Roner, K. Shahi, R.E. Bleicher, J.L. Roehr, K.D. Bassett, Synthesis of organotin polyamine ethers containing acyclovir and their preliminary anticancer and antiviral activity. J. Inorg. Organomet. Polym Mater. 16, 249–257 (2006)
G. Barot, K.R. Shahi, M.R. Roner, C.E. Carraher Jr, Synthesis, structural characterization, and ability to inhibit cancer growth of a series of organotin poly(ethylene glycols). J. Inorg. Organomet. Polym. 17, 595–603 (2007)
D. Siegmann-Louda, C. Carraher, D. Nagy, D. Snedden, J. Rosa, Polym. Mater. Sci. Eng. 89, 487 (2003)
C. Carraher, K. Morie, Polym. Mater. Sci. Eng. 91, 556 (2004)
C. Carraher, L. Lanz, J. Polym. Mater. 21, 51 (2005)
M. Roner, C. Carraher, T. Sabir, K. Shahi, J. Roehr, K. Bassett, Polym. Mater. Sci. Eng. 95, 525 (2006)
C. Carraher, Y. Ashida, G. Battin, Polym. Mater. Sci. Eng. 95, 556 (2006)
C. Carraher, T. Sabir, M. Roner, K. Shahi, R. Bleicher, J. Roehr, K. Bassett, JIOPM 16, 249 (2006)
C. Carraher, T. Sabir, C.L. Carraher, J. Polym. Mater. 23, 143 (2006)
M. Roner, C. Carraher, J. Roehr, K. Bassett, J. Polym. Mater. 23, 153 (2006)
R. Doucette, D. Siegmann-Louda, C. Carraher, A. Cardoso, Polym. Mater. Sci. Eng. 91, 564 (2004)
Y. Fu, M.J. Romero, A. Habtemariam, M.E. Snowden, L. Song, G.J. Clarkson, B. Qamar, A.M. Pizarro, P.R. Unwin, P.J. Sadler, The contrasting chemical reactivity of potent isoelectronic iminopyridine and azopyridine osmium(II) arene anticancer complexes. Chemical Sci. 3, 2485–2494 (2012)
H. Kostrhunova, J. Florian, O. Novakova, A.F.A. Peacock, P.J. Sadler, V. Brabec, DNA interactions of monofunctional organometallic osmium(II) antitumor complexes in cell-free media. J. Med. Chem. 51, 3635–3643 (2008)
S.H. van Rijt, P.J. Sadler, Current applications and future potential for bioinorganic chemistry in the development of anticancer drugs. Drug Disc. Today 14, 1089–1097 (2009)
H.S. van Rijt, H. Kostrhunova, V. Brabec, P.J. Sadler, Functionalization of osmium arene anticancer complexes with (poly)arginine: effect on cellular uptake, internalization and cytotoxicity. Bioconjugate Chem 22, 218–226 (2011)
P. Govender, F. Edafe, B.C.E. Makhubela, P.J. Dyson, B. Therrien, G.S. Smith, Neutral and cationic osmium(II)-arene metallodendrimers: synthesis, characterisation and anticancer activity. Inorg. Chim. Acta 409, 112–120 (2014)
G.M. Sulaiman, A.A.W. Mohammad, H.E. Abdul-Wahed, M.M. Ismail, Dig. J. Nanomater. Biostruct. 8, 273 (2013)
S. Moaddab, H. Ahari, D. Shahbazzadeh, A.A. Motallebi, A.A. Anvar, J. Rahman-Nya, M.R. Shokrgozar, Int. Nano. Lett. 1, 11 (2011)
W.J. Youngs, N. Robishaw, M.J. Panzner, K. Hindi, D.A. Medvetz, J. Youngs, C. Tessier, A. Ditto, Y.H. Yun, J. Bauer, D. Lindner, Treatment of breast cancer with an antitumor drug encapsulated in biodegradable polymeric nanoparticles. Nanotech Con-ference & Expo, 2, 5–8 (2009). http://www.nsti.org/7B0BF9F0-5EF5-4758-96879DFBECFADB8A/FinalDownload/DownloadId-797F313D90A1AEC1568812B3D7ADA4F9/7B0BF9F0-5EF5-4758-9687-9DFBECFADB8A/publications/Nanotech/2009/pdf/310.pdf. Acessed 7th November 2014
W.J. Youngs, A.R. Knapp, P.O. Wager, C.A. Tessier, Nanoparticle encapsulated silver carbene complexes and their antimicrobial and anticancer properties: a perspective. Dalton Trans. 41, 327–336 (2012)
H. Grunicke, W. Doppler, W. Helliger, B.J. Hermann, J. Hofmann, H. Lindner, B. Puschendorf, Tumor biochemistry as basis for advances in tumor chemotherapy. Arch Geschwulstforsch 56, 193–201 (1986)
O.S. Zhukova, I.A.V. Dobrynin, Current results and perspectives of the use of human tumor cell lines for antitumor drug screening. Vopr. Onkol. 47, 706–7099 (2001)
P.J. O’Dwyer, S.W. Johnson, T.C. in Hamilton, Cisplatin and Its Analogues, vol. 2, ed. by V.T. DeVita, S. Hellman, S.A. Rosenberg In Cancer Principles and Practice of Oncology (Lippincott-Raven, Philadelphia, 1997) pp 418–431
E.R. Jamieson, S.J. Lippard, Structure, recognition, and processing of cisplatin-DNA adducts. Chem. Rev. 99, 2467–2498 (1999)
M. Kartalou, J.M. Essigmann, Mechanism of resistance to cisplatin. Mutat. Res. 478, 23–43 (2001)
H. Cabral, N. Nishiyama, K. Kataoka, Optimization of (1,2-diamino-cyclohexane)platinum(II)-loaded polymeric micelles directed to improved tumor targeting and enhanced antitumor activity. J. Control. Release 121, 146–155 (2007)
N. Nishiyama, S. Okazaki, H. Cabral, M. Miyamoto, Y. Kato, Y. Sugiyama, K. Nishio, Y. Matsumura, K. Kataoka, Novel cisplatin-incorporated polymeric micelles can eradicate solid tumors in mice. Cancer Res. 63, 8977–8983 (2003)
H. Uchino, Y. Matsumura, T. Negishi, F. Koizumi, T. Hayashi, T. Honda, N. Nishiyama, K. Kataoka, S. Naito, T. Kakizoe, Cisplatin-incorporating polymeric micelles (NC-6004) can reduce nephrotoxicity and neurotoxicity of cisplatin in rats. Br. J. Cancer 93, 678–687 (2005)
H. Cabral, N. Nishiyama, S. Okazaki, H. Koyama, K. Kataoka, Preparation and biological properties of dichloro(1,2-diaminocyclohexane) platinum(II) (DACHPt)-loaded polymeric micelles. J. Control Release 101, 223–232 (2005)
N. Nishiyama, F. Koizumi, S. Okazaki, Y. Matsumura, K. Nishio, K. Kataoka, Differential gene expression profile between PC-14 cells treated with free cisplatin and cisplatin incorporated polymeric micelles. Bioconjug. Chem. 14, 449–457 (2003)
N. Nishiyama, Y. Kato, Y. Sugiyama, K. Kataoka, Cisplatin-loadedpolymer-metal complex micelle with time-modulated decaying property as a novel drug delivery system. Pharm. Res. 18, 1035–1041 (2001)
N. Nishiyama, M. Yokoyama, T. Aoyagi, T. Okano, Y. Sakurai, K. Kataoka, Preparation and characterization of self-assembled polymer-metal complex micelle from cis-dichlorodiammineplatinum(II) and poly(ethylene glycol)-poly(aspartic acid) block copolymer in an aqueous medium. Langmuir 15, 377–383 (1999)
Y. Mizumura, Y. Matsumura, T. Hamaguchi, N. Nishiyama, K. Kataoka, T. Kawaguchi, W.J.M. Hrushesky, F. Moriyasu, T. Kakizoe, Cisplatin-incorporated polymeric micelles eliminate nephrotoxicity, while maintaining antitumor activity. Jpn. J. Cancer Res. 92, 328–336 (2001)
V.T. Huynh, P. de Souza, M.H. Stenzel, Polymeric micelles with pendant dicarboxylato chelating ligands prepared via a michael addition for cis-platinum drug delivery. Macromolecules 44, 7888–7900 (2011)
Y. Miura, T. Takenaka, K. Toh, S. Wu, H. Nishihara, M.R. Kano, Y. Ino, T. Nomoto, Y. Matsumoto, H. Koyama, H. Cabral, N. Nishiyama, K. Kataoka, Cyclic RGD-linked polymeric micelles for targeted delivery of platinum anticancer drugs to glioblastoma through the blood brain tumor barrier. ACS Nano 7, 8583–8592 (2013)
V.B. Jadhav, Y.J. Jun, J.H. Song, M.K. Park, J.H. Oh, S.W. Chae, I.-S. Kim, S.-J. Choi, H.J. Lee, Y.S. Sohn, A novel micelle-encapsulated platinum(II) anticancer agent. J. Controll. Release 147, 144–150 (2010)
H.T.T. Duong, V.T. Huynh, P. de Souza, M.H. Stenzel, Core-cross-linked micelles synthesized by clicking bifunctional Pt(IV) anticancer drugs to isocyanates. Biomacromolecules 11, 2290–2299 (2010)
N. Malik, E.G. Evagorou, R. Duncan, Dendrimer-platinate: a novel approach to cancer chemotherapy. Anticancer Drugs 10, 767–776 (2009)
R. Duncan, N. Malik, Dendrimers: biocompatibility and potential for delivery of anticancer agents. Proc. Int. Symp. Control. Release Bioact. Mater. 23, 105–106 (1996)
E.R. Gillies, J.M.J. Fréchet, Dendrimers and dendritic polymers in drug delivery. Drug Disc. Today 10, 35–43 (2005)
G.J. Kirkpatrick, J.A. Plumb, O.B. Sutcliffe, D.J. Flint, N.J. Wheate, Evaluation of anionic half generation 3.5-6.5 poly(amidoamine) dendrimers as delivery vehicles for the active component of the anticancer drug cisplatin. J. Inorg. Biochem. 105, 1115–1122 (2011)
I. Haririan, M.S. Alavidjeh, M.R. Khorramizadeh, M.S. Ardestani, Z.Z. Ghane, H. Namazi, Anionic linear-globular dendrimer-cis-platinum (II)conjugates promote cytotoxicity in vitro against different cancer cell lines. Int. J. Nanomed. 5, 63–75 (2010)
T. Kapp, A. Dullin, R. Gust, Platinum(II)–Dendrimer conjugates: synthesis and investigations on cytotoxicity cellular distribution, platinum release, DNA, and protein binding. Bioconjugate Chem. 21, 328–337 (2010)
X. Zhao, S.C.J. Loo, P.P.-F. Lee, T.T.Y. Tan, C.K. Chu, Synthesis and cytotoxic activities of chloropyridylimineplatinum(II) and chloropyridyliminecopper(II) surface-functionalized poly(amidoamine) dendrimers. J. Inorg. Biochem. 104, 105–110 (2010)
B.A. Howell, D. Fan, Poly(amidoamine) dendrimer-supported organoplatinum antitumour agents. Proc. R. Soc. A 466, 1515–1526 (2010)
P. Zhou, Z. Li, Y. Chau, Synthesis, characterization, and in vivo evaluation of poly(ethylene oxide-co-glycidol)-platinate conjugate. Eur. J. Pharm. Sci. 41(2010), 464–472 (2010)
M.T. Johnson, E.W. Neuse, C.E.J. van Rensburg, E. Kreft, Cell growth-inhibiting properties of selected carrier-bound, monoamine-coordinated platinum(II) compounds. J. Inorg. Organomet. Polym. 13, 55–67 (2003)
J. Bariyanga, M.T. Johnson, E.M. Mmutlane, E.W. Neuse, A water-soluble polyamide containing cis-dicarboxylato-chelated platinum(II). J. Inorg. Organomet. Polym Mater. 15, 335–340 (2005)
G. Caldwell, E.W. Neuse, C.E.J. van Rensburg, Cytotoxicity of selected water-soluble polymer-cisdiaminedichloroplatinum(II) conjugates against the human HeLa cancer cell line. J. Inorg. Organomet. Polym. 7, 217–231 (1997)
G. Caldwell, E.W. Neuse, C.E.J. van Rensburg, Cytotoxic activity of two polyaspartamide-based monoamineplatinum(II) conjugates against the HeLa cancer cell line. Appl. Organomet. Chem. 13, 189–194 (1999)
M.T. Johnson, L.L. Komane, D.D. N’Da, E.W. Neuse, Polymeric drug carriers functionalized with pairwise arranged hydroxyl and/or carboxyl groups for platinum chelation. J. Appl. Polym. Sci. 96, 10–19 (2005)
E.W. Neuse, Carrier-bound platinum and ironcompounds with carcinostatic properties. Polym. Adv. Technol. 9, 786–793 (1998)
E.W. Neuse, Platinum coordination compounds in cancer research and chemotherapy. S. Afr. J. Sci. 95, 509–516 (1999)
E.W. Neuse, G. Caldwell, Cis-diaminedichloroplatinum(II) complexes reversibly linked into the main chain of water-soluble polyamides. J. Inorg. Organomet. Polym. 7, 163–181 (1997)
E.W. Neuse, G. Caldwell, A.G. Perlwitz, Carrier polymers for cisplatin-type anticancer drug models. Polym. Adv. Technol. 7, 867–872 (1996)
E.W. Neuse, N. Mphephu, H.M. Netshifhefhe, M.T. Johnson, Synthesis and preliminary in vitro evaluation of polymeric dicarboxylato- and dihydroxylatoplatinum(II) chelates as antiproliferative agents. Polym. Adv. Technol. 13, 884–895 (2002)
B. Schechter, G. Caldwell, M.G. Meirim, E.W. Neuse, Preliminary toxicological studies of selected water-soluble polymer-platinum conjugates. Appl. Organomet. Chem. 14, 701–708 (2000)
W.C. Shen, K. Beloussow, M.G. Meirim, E.W. Neuse, G. Caldwell, Antiproliferative activity of polymer-bound, monoamine-coordinated platinum complexes against LNCaP human metastatic prostate adenocarcinoma cells. J. Inorg. Organomet. Polym. 10, 51–60 (2000)
T. Smit, E.W. Neuse, P. Becker, R. Anderson, C.E.J. van Rensburg, Comparison of the effects of cisplatin and a novel platinum polymer conjugate on the production of reactive oxygen species by human neutrophils in vitro. Drug Dev. Res. 66, 204–209 (2005)
T. Smit, J.R. Snyman, E.W. Neuse, L. Bohm, C.E.J. van Rensburg, Evaluation of cisplatin and a novel platinum polymer conjugate for drug toxicity and drug distribution in mice. Anticancer Drugs 16, 501–506 (2005)
X. Lin, Q. Zhang, J.R. Rice, D.R. Stewart, D.P. Nowotnik, S.B. Howell, Improved targeting of platinum chemotherapeutics: the antitumour activity of the HPMA copolymer platinum agent AP5280 in murine tumour models. Eur. J. Cancer 40, 291–297 (2004)
J.M. Rademaker-Lakhai, C. Terret, S.B. Howell, C.M. Baud, R.F. de Boer, D. Pluim, J.H. Beijnen, J.H.M. Schellens, J.P. Droz, A phase I andpharmacological study of the platinum polymer AP5280 given as an intravenous infusion once every 3 weeks in patients with solid tumors. Clin. Cancer Res. 10, 3386–3395 (2004)
S.C. van der Schoot, B. Nuijen, P. Sood, K.B. Thurmond, D.R. Stewart, J.R. Rice, J.H. Beijnen, Pharmaceutical development, quality control, stability and compatibility of a parenteral lyophilized formulation of the investigational polymer-conjugated platinum antineoplastic agent AP534. Pharmazie 61, 835–844 (2006)
M. Campone, J.M. Rademaker-Lakhai, J. Bennouna, S.B. Howell, D.P. Nowotnik, J.H. Beijnen, J.H.M. Schellens, Phase I and pharmacokinetic trial of AP5346, a DACH-platinum-polymer conjugate, administered weekly for three out of every 4 weeks to advanced solid tumor patients. Cancer Chemother. Pharmacol. 60, 523–533 (2007)
K.J. Haxton, H.M. Burt, Polymeric drug delivery of platinum-based anticancer agents. J. Pharm. Sci. 98(2009), 2299–2316 (2009)
E. Gianasi, M. Wasil, E.G. Evagorou, A. Keddle, G. Wilson, R. Duncan, HPMA copolymer platinates as novel antitumour agents: in vitro properties, pharmacokinetics and antitumour activity in vivo. Eur. J. Cancer 35, 994–1002 (1999)
A. Furin, A. Guiotto, F. Baccichetti, G. Pasut, C. Deuschel, R. Bertani, F.M. Veronese, Synthesis, characterization and preliminary cytotoxicity assays of poly(ethylene glycol)-malonato-Pt- DACH conjugates. Eur. J. Med. Chem. 38, 739–749 (2003)
S.B. Lee, S.C. Song, J.I. Jin, Y.S. Sohn, Synthesis and antitumor activity of polyphosphazene/methoxy-poly(ethylene glycol)/(diamine)platinum(II) conjugates. Polym. J. 31, 1247–1252 (1999)
R. Song, Y.J. Jun, J.I. Kim, C. Jin, Y.S. Sohn, Synthesis, characterization, and tumor selectivity of a polyphosphazene-platinum(II) conjugate. J. Controll. Release 105, 142–150 (2005)
S.C. Song, S.B. Lee, B.H. Lee, H.W. Ha, K.T. Lee, Y.S. Sohn, Synthesis and antitumor activity of novel thermosensitive platinum(II)-cyclotriphosphazene conjugates. J. Controll. Release 90, 303–311 (2003)
S.C. Song, Y.S. Sohn, Synthesis and hydrolytic properties of polyphosphazene/(diamine)platinum/saccharide conjugates. J. Controll. Release 55, 161–170 (1998)
Y.J. Jun, J.I. Kim, M.J. Jun, Y.S. Sohn, Selective tumor targeting by enhanced permeability and retention effect. Synthesis and antitumor activity of polyphosphazene-platinum (II) conjugates. J. Inorg. Biochem. 99, 1593–1601 (2005)
Y.S. Sohn, H. Baek, Y.H. Cho, Y.A. Lee, O.S. Jung, C.O. Lee, Y.S. Kim, Synthesis and antitumor activity of novel polyphosphazene-(diamine)platinum (II) conjugates. Int. J. Pharm. 153, 79–91 (1997)
J.-Y. Fang, J.-P. Chen, Y.-L. Leu, J.-W. Hu, The delivery of platinum drugs from thermosensitive hydrogels containing different ratios of chitosan. Drug Deliv. 15, 235–243 (2008)
G. Tamasi, M. Casolaro, A. Magnani, A. Sega, L. Chiasserini, L. Messori, C. Gabbiani, S.M. Valiahdi, M.A. Jakupec, B.K. Keppler, M.B. Hursthouse, R. Cini, New platinum–oxicam complexes as anti-cancer drugs. Synthesis, characterization, release studies from smart hydrogels, evaluation of reactivity with selected proteins and cytotoxic activity in vitro. J. Inorg. Biochem. 104, 799–814 (2010)
W. Zhu, Y. Li, L. Liu, Y. Chen, C. Wang, F. Xi, Supramolecular hydrogels from cisplatin-loaded block copolymer nanoparticles and α-cyclodextrins with a stepwise delivery property. Biomacromolecules 11, 3086–3092 (2010)
M. Konishia, Y. Tabata, M. Kariya, A. Suzuki, M. Mandai, K. Nanbu, K. Takakura, S. Fujii, In vivo anti-tumor effect through the controlled release of cisplatin from biodegradable gelatin hydrogel. J. Controll. Release 92, 301–313 (2003)
M. Konishi, Y. Tabata, M. Kariya, H. Hosseinkhani, A. Suzuki, K. Fukuhara, M. Mandai, K. Takakura, S. Fujii, In vivo anti-tumor effect of dual release of cisplatin and adriamycin from biodegradable gelatin hydrogel. J. Controll. Release 103, 7–19 (2005)
J.-H. Kim, Y.-S. Kim, K. Park, S. Lee, H.Y. Nam, K.H. Min, H.G. Jo, J.H. Park, K. Choi, S.Y. Jeong, R.-W. Park, I.S. Kim, K. Kim, I. Chan, Antitumor efficacy of cisplatin-loaded glycol chitosan nanoparticles in tumor-bearing mice. J. Controll. Release 127, 41–49 (2008)
C. Mario, D.B. Barbara, M. Emilia, Hydrogel containing l-valine residues as a platform for cisplatin chemotherapy. Colloids Surf., B 88, 389–395 (2011)
A. Houlton, R.M.G. Roberts, J. Silver, J. Organomet. Chem. 418, 107–112 (1991)
D. Osella, M. Ferrali, P. Zanello, F. Laschi, M. Fontani, C. Nervi, G. Cavigiolio, Inorg. Chim. Acta 306, 42–48 (2000)
G. Caldwell, M.G. Meirim, E.W. Neuse, C. E. J.van Rensburg. Appl. Organomet. Chem. 12, 793–799 (1998)
E.W. Neuse, Polym. Adv. Technol. 9, 786–793 (1998)
E.W. Neuse, Macromol. Symp. 172, 127–138 (2001)
M.T. Johnson, E. Kreft, D.D. N’Da, E.W. Neuse, C.E.J. van Rensburg, J. Inorg. Organomet. Polym. 13, 255–267 (2003)
B.D. Nkazi, E.W. Neuse, E.R. Sadiku, B.A. Aderibigbe, Synthesis, characterization and kinetic release profile of iron containing polymeric co-conjugates with antiproliferative activity. J. Inorg. Organomet. Polym Mater. 24, 302–314 (2014)
B.D. Nkazi, E.W. Neuse, E.R. Sadiku, B.A. Aderibigbe, Synthesis, characterization, kinetic release study and evaluation of hydrazone linker in ferrocene conjugates at different pH values. J. Drug Deliv. Sci. Technol. 23, 537–545 (2013)
I.A. Mufula, B.A. Aderibigbe, E.W. Neuse, H.E. Mukaya, Macromolecular co-conjugates of methotrexate and ferrocene in the chemotherapy of cancer. J. Inorg. Organomet. Polym Mater. 22, 423–428 (2012)
A.I. Mufula, E.W. Neuse, Macromolecular carriers for methotrexate and ferrocene in cancer chemotherapy. J. Inorg. Organomet. Polym Mater. 21, 511–526 (2011)
H. Wei, C.-Y. Quan, C. Chang, X.-Z. Zhang, R.-X. Zhuo, J. Phys. Chem. B 114, 5309–5314 (2010)
N. Katsaros, A. Anagnostopoulou, Rhodium and its compounds as potential agents in cancer treatment. Crit. Rev. Oncol. Hematol. 42, 297–308 (2002)
K.S. McCully, M.P. Vezeridis, Antineoplastic activity of a rhodium trichloride complex of oxalyl homocysteine thiolactone. Cancer Invest. 5, 25–30 (1987)
R. Payne, P. Govender, B. Therrien, C.M. Clavel, P.J. Dyson, G.S. Smith, Neutral and cationic multinuclear half-sandwich rhodium and iridiumcomplexes coordinated to poly(propyleneimine) dendritic scaffolds: synthesis and cytotoxicity. J. Organomet. Chem. 729, 20–27 (2013)
L.C. Sudding, R. Payne, P. Govender, F. Edafe, C.M. Clavel, P.J. Dyson, B. Therrien, G.S. Smith, Evaluation of the in vitro anticancer activity of cyclometalated half-sandwich rhodium and iridium complexes coordinated to naphthaldimine-based poly(propyleneimine) dendritic scaffolds. J. Organomet. Chem. 774, 79–85 (2014)
I. Ott, B. Kircher, R. Dembinski, R. Gust, Alkyne hexacarbonyl dicobalt complexes in medicinal chemistry and drug development. Expert Opin. Ther. Pat. 18, 327–337 (2008)
I. Ott, K. Schmidt, B. Kircher, P. Schumacher, T. Wiglenda, R. Gust, Antitumor-active cobalt-alkyne complexes derived from acetylsalicylic acid: studies on the mode of drug action. J. Med. Chem. 48, 622–629 (2005)
C.D. Sergeant, I. Ott, A. Sniady, S. Meneni, R. Gust, A.L. Rheingold, R. Dembinski, Metallo-nucleosides: synthesis and biological evaluation of hexacarbonyl dicobalt 5-alkynyl-2′-deoxyuridines. Org. Biomol. Chem. 6, 73–80 (2008)
A.B. Withey, G. Chen, T.L. Nguyen, M.H. Stenzel, Macromolecular cobalt carbonyl complexes encapsulated in a click-cross-linked micelle structure as a nanoparticle to deliver cobalt pharmaceuticals. Biomacromolecules 10, 3215–3226 (2009)
R.S. Kumar, S. Arunachalam, V.S. Periasamy, C.P. Preethy, A. Riyasdeen, M.A. Akbarsha, Synthesis, DNA binding and antitumor activities of some novel polymer–cobalt(III) complexes containing 1,10-phenanthroline ligand. Polyhedron 27, 1111–1120 (2008)
G. Vignesh, R. Senthilkumar, P. Paul, V.S. Periasamy, M.A. Akbarsha, S. Arunachalam, Protein binding and biological evaluation of a polymer-anchored cobalt(III) complex containing a 2,2′-bipyridine ligand. RSC Adv. 4, 57483–57492 (2014)
E. Sabbioni, G. Pozzi, S. Devos, A. Pintar, L. Casella, M. Fischbach, The intensity of vanadium(V)-induced cytotoxicity and morphological transformation in BALB/3T3 cells is dependent on glutathione-mediated bioreduction to vanadium(IV). Carcinogenesis 14, 2565–2568 (1993)
A. Stem, X. Yin, S.S. Tsang, A. Davison, J. Moon, Vanadium as a modulator of cellular regulatory cascades and oncogene expression. Biochem. Cell Biol. 71, 103–112 (1993)
A. Chakraborty, R. Ghosh, K. Roy, S. Ghosh, P. Chowdhury, M. Chatterjee, Vanadium: a modifier of drug-metabolizing enzyme patterns and its critical role in cellular proliferation in transplantable murine lymphoma. Oncology 52, 310–314 (1995)
H.J. Thompson, N.D. Chasteen, L.D. Meekr, Dietary vanadyl(IV) sulphate inhibits chemically-induced mammary carcinogenesis. Carcinogenesis 5, 849–851 (1984)
A. Bishayee, M. Chatterjee, Inhibitory effect of vanadium on rat liver carcinogenesis initiated with diethylnitrosamine and promoted by phenobarbital. Br. J. Cancer 71, 1214–1220 (1995)
S. Sardar, A. Mondal, M. Chatterjee, Protective role of vanadium in the survival of hosts during the growth of a transplantable murine lymphoma and its profound effects on the rates and patterns of biotransformation. Neoplasma 40, 27–30 (1993)
T.F. Cruz, A. Morgan, W. Min, In vitro and in vivo antineoplastic effects of orthovanadate. Mol. Cell Biochem. 153, 161–166 (1995)
A.M. Evangelou, Vanadium in cancer treatment. Crit. Rev. Oncol./Hematol. 42, 249–265 (2002)
J.K. Jackson, W. Min, T.F. Cruz, S. Cindric, L. Arsenault, D.D. Von Hoff, D. Degan, W.L. Hunter, H.M. Burt, A polymer-based drug delivery system for the antineoplastic agent bis(maltolato)oxovanadium in mice. Br. J. Cancer 75, 1014–1020 (1997)
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is written in memory of late Prof. E.W. Neuse (em) who dedicated his research towards the design and characterization of polymer-drug conjugates containing metal based anticancer drugs.
Rights and permissions
About this article
Cite this article
Aderibigbe, B.A. Polymeric Prodrugs Containing Metal-Based Anticancer Drugs. J Inorg Organomet Polym 25, 339–353 (2015). https://doi.org/10.1007/s10904-015-0220-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-015-0220-7