Abstract
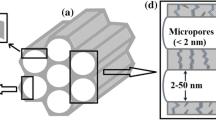
Zeolite honeycomb monoliths were prepared from ZSM-5 powders synthesized under hydrothermal conditions using microporous silica obtained by selective leaching of metakaolinite. This honeycomb material was compared with those prepared using alkoxides (TEOS) as the silica source. The honeycomb monoliths were formed by extrusion of paste made from the synthesized powders through a multi-channel honeycomb die. The morphology and porous properties of these materials were studied using XRD, FTIR, SEM and N2/Ar adsorption. ZSM-5 grains in the monoliths prepared from metakaolinite showed platy morphology with preferred orientation of the crystals in the extruded surface, and displayed an absence of secondary growth. The twinned morphology of ZSM-5 crystals was observed in the monoliths prepared using TEOS and this contributed to an increase in the external surface area even though the total surface area was identical to that of samples prepared from metakaolinite. The physical properties, thermal stability and mechanical strength of the monoliths was compared with zeolite-coated honeycombs. The results show that microporous silica prepared by acid leaching of metakaolinite is a cost-effective raw material for preparing ZSM-5 honeycomb monoliths with controlled morphology and tunable SiO2/Al2O3 ratios.
Similar content being viewed by others
References
K. Otto, C.N. Montreuil, O. Todor, R.W. McCabe, and H.S. Gandhi, Ind. Eng. Chem. Res. 30, 2333 (1991).
W. Held, A. Konig, T. Richter, and L.Puppe, SAE 900496, 209 (1990).
M. Iwamoto, S. Yokoo, K. Sakai, and S. Kagawa, J. Chem. Soc., Faraday Trans. 1–77, 1629 (1981).
J.L. Williams, Catalysis Today 69, 3 (2001).
C.D. Madhusoodana, R.N. Das, Y. Kameshima, A. Yasumori and K. Okada, Micropor. Mesopor. Mater. 46, 249 (2001).
F. Mizukami, Std. Surf. Sci. Cat. 125, 1 (1999).
J. Hedlund, S. Mintova, and J. Sterte, Micropor. Mesopor. Mater. 28, 185 (1999).
I.M. Lachman and J.L. Williams, Catalysis Today 14, 317 (1992).
Y.Y. Li, S.P. Perera, B.D. Crittenden, and J. Bridgwater, Powder Technology 116, 85 (2001).
T. Kato, Material Integration 13, 31 (2000).
Y. S. Bhat, J. Das, and A. B. Halgeri, App. Catalysis, A: Gen 122, 161 (1995).
P.A. Jacobs and J.A. Martens, Synthesis of High Silica Aluminosilicate Zeolites (Elsevier, Amsterdam, 1987).
D.W. Breck, Zeolite Molecular Sieves Structure, Chemistry and Uses (John Wiley {&} Sons, Inc., New York, 1974).
R.M. Barrer, Hydrothermal Chemistry of Zeolites (Academic Press, London, 1982).
M. Murat, A. Amokrane, J.P. Bastide, and L. Montanaro, Clay Min. 27, 119 (1992).
D. Akolekar, A. Chafee, and R.F. Howe, Zeolites 19, 359 (1997).
S. Chandrasekhar and P.N. Pramada, J. Porous Mater. 6, 283 (1999).
S. Chandrasekhar and P. N. Pramada, Ceramics International 28, 277 (2002).
F.G. Dwyer. and A.B. Schwartz, US Patent 4,091,007 (1978).
British Patent GB 2017520 assigned to Engelhard Min. {&} Chem. (1979).
K. Okada, A. Shimai, T. Takei, S. Hayashi. A. Yasumori, and K.J.D. MacKenzie, Micropor. Mesopor. Mater. 21, 289 (1998).
C.D. Madhusoodana, Y. Kameshima, A. Yasumori, and K. Okada, Clay Science 11, 369 (2001).
A. Saito and H.C. Foley, AIChE.J 37, 429 (1991).
C.D. Madhusoodana, R.N. Das, A.M. Umarji, and K. Okada, Ceramic Trans. 112, 539 (2001).
J.P. Bellat, O. Bertrand, F. Bouvier, M. Broyer, V. Francois, S. Maure, and G. Weber, Std. Surf. Sci. Cat. 125, 737 (1999).
C.D. Madhusoodana, R.N. Das, Y. Kameshima, and K. Okada, Trans. Mat. Res. Soc. Japan. 29, 2293 (2004).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Madhusoodana, C.D., Das, R.N., Kameshima, Y. et al. Preparation of ZSM-5 Zeolite Honeycomb Monoliths Using Microporous Silica Obtained from Metakaolinite. J Porous Mater 12, 273–280 (2005). https://doi.org/10.1007/s10934-005-3125-y
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/s10934-005-3125-y