Abstract
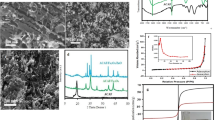
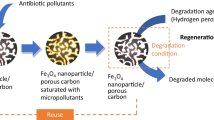
In the present work, the adsorption performance of powdered activated carbon magnetized by iron(III) oxide magnetic nanoparticles (PAC@Fe3O4-MN) for the removal of ciprofloxacin (CIP) was extensively studied using batch experiments. First, PAC@Fe3O4-MN was synthesized and prepared by co-precipitation method, then it was subjected to characterization study using advanced techniques. Then, the adsorption ability of PAC@Fe3O4-MN for CIP was determined at different initial CIP concentration (10–100 mg/L), pH (3–11), PAC@Fe3O4-MN dose (0.1–0.6 g/L), shaking speed (50–300 rpm), contact time (0–120 min) and temperature (283–328 K). Results showed that PAC@Fe3O4-MN possessed excellent adsorptive properties and had a high practical utility. It was found that PAC particles were partially covered during the magnetization by Fe3O4-MN whereas the surface and morphological properties of both were detected in the characterization analysis of PAC@Fe3O4-MN. Values of the thermodynamic and isotherm parameters (negative \(\Delta {G}^{o}\); positive \(\Delta {H}^{o}\), and values of KF and B) indicated that CIP adsorption process onto PAC@Fe3O4-MN was favorable, spontaneous and endothermic. Kinetic and isotherm studies manifested that the interaction of CIP with PAC@Fe3O4-MN occurs via both chemical and physical reactions onto a single and homogeneous layer of active sites of PAC@Fe3O4-MN. Furthermore, the rate-controlling step of the kinetic reaction is dominated and controlled by film diffusion for all CIP concentrations that studied. PAC@Fe3O4-MN possessed an excellent adsorption capacity for CIP (109.833 mg/g at pH 7, PAC@Fe3O4-MN dose = 1 g/L, shaking speed = 200 rpm, initial CIP concentration = 100 mg/L, contact time = 60 min, and temperature = 298 K). Finally, the used adsorbent appeared to be sustainable and cost-effective for treatment of CIP laden wastewater, as it can be successfully recycled up to eight consecutive adsorption–desorption cycles.












Similar content being viewed by others
References
N. Nasseh, T.J. Al-Musawi, M.R. Miri, S. Rodriguez-Couto, A. Hossein Panahi, A comprehensive study on the application of FeNi3@SiO2@ZnO magnetic nanocomposites as a novel photo-catalyst for degradation of tamoxifen in the presence of simulated sunlight. Environ. Pollut. 261, 114127 (2020)
W. Liu, H. Xie, J. Zhang, C. Zhang, Sorption removal of cephalexin by HNO3 and H2O2 oxidized activated carbons. Sci. China Chem. 55(9), 1959–1967 (2012)
D.C. Speksnijder, D.J. Mevius, C.J. Bruschke, J.A. Wagenaar, Reduction of veterinary antimicrobial use in the Netherlands. The Dutch success model, zoonoses. Public Health 62(Suppl 1), 79–87 (2015)
K.L. Tang, N.P. Caffrey, D.B. Nóbrega, S.C. Cork, P.E. Ronksley, H.W. Barkema, A.J. Polachek, H. Ganshorn, N. Sharma, J.D. Kellner, W.A. Ghali, Restricting the use of antibiotics in food-producing animals and its associations with antibiotic resistance in food-producing animals and human beings: a systematic review and meta-analysis. Lancet Planet Health. 1(8), e316–e327 (2017)
A. Gulkowska, H.W. Leung, M.K. So, S. Taniyasu, N. Yamashita, L.W.Y. Yeung, B.J. Richardson, A.P. Lei, J.P. Giesy, P.K.S. Lam, Removal of antibiotics from wastewater by sewage treatment facilities in Hong Kong and Shenzhen, China. Water Res. 42(1), 395–403 (2008)
A.A. Mohammed, M.A. Atiya, M.A. Hussein, Studies on membrane stability and extraction of ciprofloxacin from aqueous solution using pickering emulsion liquid membrane stabilized by magnetic nano-Fe2O3. Colloids Surf. A Physicochem. Eng. Asp. 585, 124044 (2020)
K. Kümmerer, Antibiotics in the aquatic environment—a review—part I. Chemosphere 75(4), 417–434 (2009)
M.M. Soori, E. Ghahramani, H. Kazemian, T.J. Al-Musawi, M. Zarrabi, Intercalation of tetracycline in nano sheet layered double hydroxide: an insight into UV/VIS spectra analysis. J Taiwan Inst Chem Eng. 63, 271–285 (2016)
A. Mohseni-Bandpi, T.J. Al-Musawi, E. Ghahramani, M. Zarrabi, S. Mohebi, S.A. Vahed, Improvement of zeolite adsorption capacity for cephalexin by coating with magnetic Fe3O4 nanoparticles. J. Mol. Liq. 218, 615–624 (2016)
Z. Aksu, Ö. Tunç, Application of biosorption for penicillin G removal: comparison with activated carbon. Process Biochem. 40(2), 831–847 (2005)
M. Amini, M. Khanavi, A. Shafiee, Simple high-performance liquid chromatographic method for determination of ciprofloxacin in human plasma. Iran. J. Pharm. Sci. 3(2), 99–101 (2010)
J.B. Parsa, T.M. Panah, F.N. Chianeh, Removal of ciprofloxacin from aqueous solution by a continuous flow electro-coagulation process. Korean J. Chem. Eng. 33(3), 893–901 (2016)
S. Ahmadi, A. Banach, F.K. Mostafapour, D. Balarak, Study survey of cupric oxide nanoparticles in removal efficiency of ciprofloxacin antibiotic from aqueous solution: adsorption isotherm study. Desalin. Water Treat. 89, 297–303 (2017)
D. Balarak, F. Mostafapour, H. Azarpira, Adsorption isotherm studies of tetracycline antibiotics from aqueous solutions by maize stalks as a cheap biosorbent. Int. J. Pharm. Technol. 8(3), 16664–16675 (2016)
P.-H. Chang, Z. Li, J.-S. Jean, W.-T. Jiang, C.-J. Wang, K.-H. Lin, Adsorption of tetracycline on 2:1 layered non-swelling clay mineral illite. Appl. Clay Sci. 67–68, 158–163 (2012)
J. Gao, J.A. Pedersen, Adsorption of sulfonamide antimicrobial agents to clay minerals. Environ. Sci. Technol. 39(24), 9509–9516 (2005)
H. Azarpira, D. Balarak, Rice husk as a biosorbent for antibiotic metronidazole removal: Isotherm studies and model validation. Int. J. Chemtech Res. 9(7), 566–573 (2016)
D. Balarak, F.K. Mostafapour, Photocatalytic degradation of amoxicillin using UV/Synthesized NiO from pharmaceutical wastewater. Indonesian J. Chem. 19(1), 211–218 (2019)
M.-F. Li, Y.-G. Liu, S.-B. Liu, D. Shu, G.-M. Zeng, X.-J. Hu, X.-F. Tan, L.-H. Jiang, Z.-L. Yan, X.-X. Cai, Cu(II)-influenced adsorption of ciprofloxacin from aqueous solutions by magnetic graphene oxide/nitrilotriacetic acid nanocomposite: competition and enhancement mechanisms. Chem. Eng. J. 319, 219–228 (2017)
K. Yaghmaeian, G. Moussavi, A. Alahabadi, Removal of amoxicillin from contaminated water using NH4Cl-activated carbon: Continuous flow fixed-bed adsorption and catalytic ozonation regeneration. Chem. Eng. J. 236, 538–544 (2014)
R. Ding, P. Zhang, M. Seredych, T.J. Bandosz, Removal of antibiotics from water using sewage sludge- and waste oil sludge-derived adsorbents. Water Res. 46(13), 4081–4090 (2012)
S. Guiza, Biosorption of heavy metal from aqueous solution using cellulosic waste orange peel. Ecol. Eng. 99, 134–140 (2017)
S. Guiza, L. Franckb, M. Baganéa, Adsorption of dyes from aqueous solution under batch mode using cellulosic orange peel waste. Desalin. Water Treat. 113, 262–269 (2018)
T.X. Bui, H. Choi, Adsorptive removal of selected pharmaceuticals by mesoporous silica SBA-15. J. Hazard Mater. 168(2–3), 602–608 (2009)
S.M. Al-Jubouri, D.A. de Haro-Del Rio, A. Alfutimie, N.A. Curry, S.M. Holmes, Understanding the seeding mechanism of hierarchically porous zeolite/carbon composites. Microporous Mesoporous Mater. 268, 109–116 (2018)
A.H. Mahvi, F.K. Mostafapour, D. Balarak, Biosorption of tetracycline from aqueous solution by azolla filiculoides: equilibrium kinetic and thermodynamics studies. Fresenius Environ. Bull. 27(8), 5759–5767 (2018)
M.-H. To, P. Hadi, C.-W. Hui, C.S.K. Lin, G. McKay, Mechanistic study of atenolol, acebutolol and carbamazepine adsorption on waste biomass derived activated carbon. J. Mol. Liq. 241, 386–398 (2017)
S.T. Danalıoğlu, ŞS. Bayazit, Ö. Kerkez Kuyumcu, M.A. Salam, Efficient removal of antibiotics by a novel magnetic adsorbent: Magnetic activated carbon/chitosan (MACC) nanocomposite. J. Mol. Liq. 240, 589–596 (2017)
S. Li, X. Zhang, Y. Huang, Zeolitic imidazolate framework-8 derived nanoporous carbon as an effective and recyclable adsorbent for removal of ciprofloxacin antibiotics from water. J. Hazard. Mater. 321, 711–719 (2017)
F. Wang, B. Yang, H. Wang, Q. Song, F. Tan, Y. Cao, Removal of ciprofloxacin from aqueous solution by a magnetic chitosan grafted graphene oxide composite. J. Mol. Liq. 222, 188–194 (2016)
S. Qu, J. Wang, J. Kong, P. Yang, G. Chen, Magnetic loading of carbon nanotube/nano-Fe3O4 composite for electrochemical sensing. Talanta 71(3), 1096–1102 (2007)
A.A. Mohammed, F. Brouers, S. Isra’a Sadi, T.J. Al-Musawi, Role of Fe3O4 magnetite nanoparticles used to coat bentonite in zinc(II) ions sequestration. Environ. Nanotechnol. Monit. Manag. 10, 17–27 (2018)
B. Kakavandi, A. Jonidi, R. Rezaei, S. Nasseri, A. Ameri, A. Esrafily, Synthesis and properties of Fe3O4-activated carbon magnetic nanoparticles for removal of aniline from aqueous solution: equilibrium, kinetic and thermodynamic studies. IJEHSE. 10(1), 19 (2013)
A.J. Jafari, B. Kakavandi, R.R. Kalantary, H. Gharibi, A. Asadi, A. Azari, A.A. Babaei, A. Takdastan, Application of mesoporous magnetic carbon composite for reactive dyes removal: process optimization using response surface methodology. Korean J Chem Eng. 33(10), 2878–2890 (2016)
M. Yegane Badi, A. Azari, H. Pasalari, A. Esrafili, M. Farzadkia, Modification of activated carbon with magnetic Fe3O4 nanoparticle composite for removal of ceftriaxone from aquatic solutions. J. Mol. Liq. 261, 146–154 (2018)
M. Badi, A. Azari, A. Esrafili, E. Ahmadi, M. Gholami, Performance evaluation of magnetized multiwall carbon nanotubes by iron oxide nanoparticles in removing fluoride from aqueous solution. J. Mazandaran Univ. Med. Sci. 25(124), 128–142 (2015)
Y. Sun, H. Li, G. Li, B. Gao, Q. Yue, X. Li, Characterization and ciprofloxacin adsorption properties of activated carbons prepared from biomass wastes by H3PO4 activation. Bioresource Technol. 217, 239–244 (2016)
M. Khodadadi, T.J. Al-Musawi, M. Kamranifar, M.H. Saghi, A. Hossein Panahi, A comparative study of using barberry stem powder and ash as adsorbents for adsorption of humic acid. Environ. Sci. Pollut. Res. 26(25), 26159–26169 (2019)
A.A. Mohammed, T.J. Al-Musawi, S.L. Kareem, M. Zarrabi, A.M. Al-Ma’abreh, Simultaneous adsorption of tetracycline, amoxicillin, and ciprofloxacin by pistachio shell powder coated with zinc oxide nanoparticles. Arab. J. Chem. 13(3), 4629–4643 (2020)
K. Zare, V.K. Gupta, O. Moradi, A.S.H. Makhlouf, M. Sillanpää, M.N. Nadagouda, H. Sadegh, R. Shahryari-ghoshekandi, A. Pal, Z.-J. Wang, I. Tyagi, M. Kazemi, A comparative study on the basis of adsorption capacity between CNTs and activated carbon as adsorbents for removal of noxious synthetic dyes: a review. J Nanostruct Chem. 5(2), 227–236 (2015)
C.-L. Zhang, F. Zhao, Y. Wang, Thermodynamics of the solubility of ciprofloxacin in methanol, ethanol, 1-propanol, acetone, and chloroform from 293.15 to 333.15K. J. Mol. Liq. 156(2), 191–193 (2010)
A. Al-Fatesh, A. Fakeeha, Effects of calcination and activation temperature on dry reforming catalysts. J. Saudi Chem. Soc. 16(1), 55–61 (2012)
J. Ma, J. Chu, L. Qiang, J. Xue, Effect of different calcination temperatures on the structural and photocatalytic performance of Bi-TiO2/SBA-15. Int. J. Photoenergy. 2013, 875456 (2013)
Z.-X. Chen, X.-Y. Jin, Z. Chen, M. Megharaj, R. Naidu, Removal of methyl orange from aqueous solution using bentonite-supported nanoscale zero-valent iron. J. Colloid Interface Sci. 363(2), 601–607 (2011)
B. Kakavandi, A. Esrafili, A. Mohseni-Bandpi, A. Jonidi Jafari, R. Rezaei Kalantary, Magnetic Fe3O4@C nanoparticles as adsorbents for removal of amoxicillin from aqueous solution. Water Sci Technol. 69(1), 147–155 (2014)
R. Egerton, Physical Principles of Electron Microscopy (Springer, Berlin, 2005).
H. Liu, W. Liu, J. Zhang, C. Zhang, L. Ren, Y. Li, Removal of cephalexin from aqueous solutions by original and Cu(II)/Fe(III) impregnated activated carbons developed from lotus stalks Kinetics and equilibrium studies. J. Hazard. Mater. 185(2), 1528–1535 (2011)
I.S.I.S.C.O.C. Zawadzki, J. Chem. Phys. Carbon 21, 147–386 (1989)
L. Zhang, X. Song, X. Liu, L. Yang, F. Pan, J. Lv, Studies on the removal of tetracycline by multi-walled carbon nanotubes. Chem. Eng. J. 178, 26–33 (2011)
D. Balarak, F. Mostafapour, E. Bazrafshan, T.A. Saleh, Studies on the adsorption of amoxicillin on multi-wall carbon nanotubes. Water Sci. Technol. 75(7–8), 1599–1606 (2017)
V.K. Upadhyayula, S. Deng, M.C. Mitchell, G.B. Smith, Application of carbon nanotube technology for removal of contaminants in drinking water: a review. Sci. Total Environ. 408(1), 1–13 (2009)
A.I. Alwared, T.J. Al-Musawi, L.F. Muhaisn, A.A. Mohammed, The biosorption of reactive red dye onto orange peel waste: a study on the isotherm and kinetic processes and sensitivity analysis using the artificial neural network approach. Environ. Sci. Pollut. Res. 28(3), 2848–2859 (2021)
M. Dutta, N.N. Dutta, K.G. Bhattacharya, Aqueous phase adsorption of certain beta-lactam antibiotics onto polymeric resins and activated carbon. Sep. Purif. Technol. 16(3), 213–224 (1999)
M. Erşan, E. Bağda, E. Bağda, Investigation of kinetic and thermodynamic characteristics of removal of tetracycline with sponge like, tannin based cryogels. Colloids Surf B Biointerfaces. 104, 75–82 (2013)
S.-X. Zhao, N. Ta, X.-D. Wang, Effect of temperature on the structural and physicochemical properties of biochar with apple tree branches as feedstock material. Energies. 10(9), 1293 (2017)
Y. Gao, Y. Li, L. Zhang, H. Huang, J. Hu, S.M. Shah, X. Su, Adsorption and removal of tetracycline antibiotics from aqueous solution by graphene oxide. J. Colloid Interface Sci. 368(1), 540–546 (2012)
U.A. Guler, M. Sarioglu, Removal of tetracycline from wastewater using pumice stone: equilibrium, kinetic and thermodynamic studies. J Environ Health Sci Eng. 12, 79–79 (2014)
A.A. Mohammed, A.A. Najim, T.J. Al-Musawi, A.I. Alwared, Adsorptive performance of a mixture of three nonliving algae classes for nickel remediation in synthesized wastewater. J. Environ. Health Sci. Eng. 17(2), 529–538 (2019)
S. Carabineiro, T. Thavorn-Amornsri, M. Pereira, J. Figueiredo, Adsorption of ciprofloxacin on surface-modified carbon materials. Water Res. 45(15), 4583–4591 (2011)
F. Yu, Y. Li, S. Han, J. Ma, Adsorptive removal of antibiotics from aqueous solution using carbon materials. Chemosphere 153, 365–385 (2016)
H. Azarpira, Y. Mahdavi, O. Khaleghi, D. Balarak, Thermodynamic studies on the removal of metronidazole antibiotic by multi-walled carbon nanotubes. Der Pharm lettre. 8(11), 107–113 (2016)
F. Brouers, T.J. Al-Musawi, Brouers-Sotolongo fractal kinetics versus fractional derivative kinetics: a new strategy to analyze the pollutants sorption kinetics in porous materials. J. Hazard. Mater. 350, 162–168 (2018)
T.J. Al-Musawi, F. Brouers, M. Zarrabi, Kinetic modeling of antibiotic adsorption onto different nanomaterials using the Brouers-Sotolongo fractal equation. Environ. Sci. Pollut. Res. 24(4), 4048–4057 (2017)
T.J. Al-Musawi, F. Brouers, M. Zarrabi, R. Noroozi, What can the use of well-defined statistical functions of pollutants sorption kinetics teach us? A case study of cyanide sorption onto LTA zeolite nanoparticles. Environ. Technol. Innov. 10, 46–54 (2018)
S. Guiza, H. Hajji, M. Bagane, External mass transport process during the adsorption of fluoride from aqueous solution by activated clay. C. R. Chim. 22(2), 161–168 (2019)
F. Brouers, T.J. Al-Musawi, The use of the Brouers-Sotolongo fractal kinetic equation for the study of drug release. Adsorption. 26(6), 843–853 (2020)
Y.S. Ho, G. McKay, Pseudo-second order model for sorption processes. Process Biochem. 34(5), 451–465 (1999)
D. Balarak, F. Mostafapour, H. Azarpira, Adsorption kinetics and equilibrium of ciprofloxacin from aqueous solutions using corylus avellana (Hazelnut) activated carbon. Brit. J. Pharm. Res. 13(3), 1–14 (2016)
J. Pan, H. Yao, W. Guan, H. Ou, P. Huo, X. Wang, X. Zou, C. Li, Selective adsorption of 2,6-dichlorophenol by surface imprinted polymers using polyaniline/silica gel composites as functional support: Equilibrium, kinetics, thermodynamics modeling. Chem. Eng. J. 172(2), 847–855 (2011)
S. Guiza, F. Brouers, M. Bagane, Fluoride removal from aqueous solution by montmorillonite clay: kinetics and equilibrium modeling using new generalized fractal equation. Environ. Technol. Innov. (2020). https://doi.org/10.1016/j.eti.2020.101187
S.M. Al-Jubouri, N.A. Curry, S.M. Holmes, Hierarchical porous structured zeolite composite for removal of ionic contaminants from waste streams and effective encapsulation of hazardous waste. J. Hazard. Mater. 320, 241–251 (2016)
A.A. Mohammed, F.I. Abed, T.J. Al-Musawi, Biosorption of Pb(II) from aqueous solution by spent black tea leaves and separation by flotation. Desalin. Water Treat. 57(5), 2028–2039 (2016)
S.M. Al-Jubouri, S.M. Holmes, Immobilization of cobalt ions using hierarchically porous 4A zeolite-based carbon composites: Ion-exchange and solidification. J. Water Process. Eng. 33, 101059 (2020)
F. Brouers, T.J. Al-Musawi, On the optimal use of isotherm models for the characterization of biosorption of lead onto algae. J. Mol. Liq. 212, 46–51 (2015)
F.H. Kamar, A.C. Nechifor, G. Nechifor, T.J. Al-Musawi, A.H. Mohammed, Aqueous phase biosorption of Pb (II), Cu (II), and Cd (II) onto cabbage leaves powder. Int. J. Chem. React. Eng. (2017). https://doi.org/10.1515/ijcre-2015-0178
D. Balarak, T.J. Al-Musawi, I.A. Mohammed, H. Abasizadeh, The eradication of reactive black 5 dye liquid wastes using Azolla filiculoides aquatic fern as a good and an economical biosorption agent. SN Appl. Sci. 2(6), 1015 (2020)
M.H. Alhassani, S.M. Al-Jubouri, H.A. Al-Jendeel, Stabilization of phenol trapped by agricultural waste: a study of the influence of ambient temperature on the adsorbed phenol. Desalin Water Treat. 187, 266–276 (2020)
N. Nasseh, R. Khosravi, G.A. Rumman, M. Ghadirian, H. Eslami, M. Khoshnamvand, T.J. Al-Musawi, A. Khosravi, Adsorption of Cr(VI) ions onto powdered activated carbon synthesized from Peganum harmala seeds by ultrasonic waves activation. Environ. Technol. Innov. 21, 101277 (2021)
W. Liu, J. Zhang, C. Zhang, L. Ren, Sorption of norfloxacin by lotus stalk-based activated carbon and iron-doped activated alumina: Mechanisms, isotherms and kinetics. Chem. Eng. J. 171(2), 431–438 (2011)
Y. Zhao, F. Tong, X. Gu, C. Gu, X. Wang, Y. Zhang, Insights into tetracycline adsorption onto goethite: experiments and modeling. Sci. Total Environ. 470–471, 19–25 (2014)
C. Nguyen, D.D. Do, The Dubinin-Radushkevich equation and the underlying microscopic adsorption description. Carbon 39(9), 1327–1336 (2001)
N. Dhiman, N. Sharma, Batch adsorption studies on the removal of ciprofloxacin hydrochloride from aqueous solution using ZnO nanoparticles and groundnut (Arachis hypogaea) shell powder: a comparison. Indian Chem. Eng. (2018). https://doi.org/10.1080/00194506.2018.1424044
N. Khoshnamvand, S. Ahmadi, F.K. Mostafapour, Kinetic and isotherm studies on ciprofloxacin an adsorption using magnesium oxide nanopartices. J. Appl. Pharm. Sci. 7(11), 79–83 (2017)
Acknowledgements
This work was supported by Zahedan University of Medical Sciences, Zahedan, Iran.
Author information
Authors and Affiliations
Contributions
TJA-M: Writing, Review, Editing, and Supervision. AHM: Writing, Results, Discussion, and Experiments. ADK: Methodology, and Data analysis. DB: Editing, Methodology, Data analysis, and Project administration.
Corresponding author
Ethics declarations
Competing of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the study reported in this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Al-Musawi, T.J., Mahvi, A.H., Khatibi, A.D. et al. Effective adsorption of ciprofloxacin antibiotic using powdered activated carbon magnetized by iron(III) oxide magnetic nanoparticles. J Porous Mater 28, 835–852 (2021). https://doi.org/10.1007/s10934-021-01039-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-021-01039-7