Abstract
The polycrystalline perovskites La2/3(Ca1−x Sr x )1/3MnO3 (x=0,0.2,0.4,0.6,0.8,1) were successfully prepared by a modified method of solid-state reactions, in which the ingredient mixture with ethanol as a liquid milling medium to form suspension was milled by high-energy ball milling for 10 h and sintered in air at 1400 °C for 10 h. The microstructure, electrical transport, and low-field magnetoresistance (LFMR) of the perovskites were investigated to study the room-temperature magnetoresistance (RTMR) behavior. The results reveal that the metal-to-insulator transition temperature (T MI) increased with increasing doping level x, and the peak values of the magnetoresistance (MR) near T MI dropped with the more Ca2+ substituted. A single-phase La2/3Ca1/3MnO3 showed T MI at 263 K and the peak MR of 23 % in the applied field of 3 kOe near T MI. The LFMR effect at room temperature could be obtained by controlling the doping Sr2+-substituted Ca2+ level. When x=0.29, transition temperature (T MI) was 305.30 K, and the MR effect was recorded up to 12.9 % at 298.55 K and 3 kOe. Finally, the possible mechanism is discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Intense researches have been carried out for the potential application of magnetic field sensor and magnetic recording, since large magnetoresistance (MR) effect was discovered in the doped manganese oxide Re1−x A x MnO3 (Re=trivalent rare earth and A=divalent metal). This kind of perovskite possesses unique properties as the colossal magnetoresistance (CMR), metal-to-insulator (M-I) transition and phase separation, etc. The Double Exchange (DE) mechanism was first put forward to explain the electron transport property [1, 2]. Later researches on the phase diagram [3], oxygen influence [4, 5], charge ordering [6], Jahn–Teller effect [7, 8], and grain boundary and lattice effect [9–11] have made contribution to understanding the mechanism of the doped rare earth manganese perovskite. The CMR usually occurs at low temperature range and strong magnetic field, and this has become the main obstacle to the practical application. Nowadays, researchers are focusing on how to obtain a large value of the MR at a low field and room temperature in order to satisfy practical applications. The LFMR [12–15], which is the significant MR effect that can be observed at a low magnetic field just several thousand mT or below, has been triggered renewal researches on the polycrystalline doped rare earth manganese perovskite. The LFMR is usually observed at low temperature and believed to be aroused by the spin-dependent scattering [16, 17] or spin-dependent tunneling [18–20] on the grain boundaries. The transport property is sensitive to the characteristics of grain boundaries, so samples with smaller grain will exhibit larger LFMR. This recalls people to restudy the synthesis of manganese perovskite.
As we know, the magnetic-ordering temperature T C of La1−x Ca x MnO3 (LCMO) is below room temperature at about 270 K [21], while T C of La1−x Sr x MnO3 (LSMO) is far above the room temperature, and the highest temperature is about 360 K [22]. At the transition temperature, the MR value of LCMO is relatively greater than that of LSMO. The nature of ferromagnetic phase transition and its critical properties in La0.7Ca0.3−x Sr x MnO3 (x=0,0.05,0.1,0.2, and 0.25) single crystals have been studied systematically by Phana et al. [23]. So we can change the value of x in La2/3(Ca1−x Sr x )1/3MnO3 to systematically study the room-temperature MR effect.
In this paper, we have employed high-energy ball milling to refine the ingredient mixture by adding ethanol as a liquid milling medium to form suspension before milling and heat treatment [24]. This method is different from the traditional mechanical alloying [25, 26] and mechanochemical processing [27], and we only intend to reduce the size of the particles by ball milling but not any amorphization or chemical reactions [28]. Ethanol can absorb some of the heat that is generated due to the milling process, as well as separate the new formed surface of ground powders to avoid recombination or reactions. Simultaneously, powders can suffer more profound milling, which was helpful for obtaining the smaller particle size and avoiding the inhomogeneity to be introduced. By this method, the polycrystalline perovskites La2/3(Ca1−x Sr x )1/3MnO3 (LCSMO) (x=0,0.2,0.4,0.6,0.8,1) were successfully synthesized. The microstructure and transportation of LCSMO have been investigated. We have also discussed the relation between the peak MR value, the metal-to-insulator transition temperature (T MI), and doped level of x.
2 Experimental
Stoichiometric amount of commercial La(OH)3, CaCO3, SrCO3, and MnO2 powders were mixed according to formula La2/3(Ca1−x Sr x )1/3MnO3 (x=0,0.2,0.4,0.6,0.8,1) with ethanol and milled by a planetary ball miller. The milling time (t m) was set to be 10 h. The as-milled powders were dried at 90 °C and pressed into pieces, followed by sintering process at 1400 °C for 10 h in air [24].
Both of the as-milled powder and the ceramic samples were characterized by X-ray diffraction (XRD) to confirm the crystalline phase. Scanning electron microscopy (SEM) was employed to examine the microstructure of samples. The electrical and magnetic transport behaviors were measured by the standard dc four-probe method in the temperature range from 50 to 400 K at an external field of 3 kOe.
3 Results and Discussions
Figure 1 shows the XRD patterns of the starting materials and the as-milled powders. The average diameter of as-milled powder was about 90 nm by the classical Sheerer formula calculation. Since no new diffraction peaks came up, it could be seen that not any amorphization or chemical reactions occurred, and the as-milled powders still remained in their starting composition. The improved ball milling method with ethanol as a liquid ingredient mixture medium to form suspension is helpful for reducing particle size and obtaining homogeneous powders.
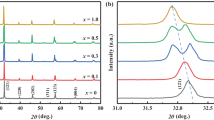
From Fig. 2 we can see that all of the heat treated samples have crystallized into single-phase perovskites, and this means that the doped lanthanum manganese perovskites have been successfully synthesized by this modified method.
Figure 3 displays the SEM result of La2/3Ca1/3MnO3. The morphology of this ceramic was identical with those synthesized by solid-state reaction. We can find out that the well-crystallized grain sizes of the ceramics were uniformly in the range of 4–5 μm.
Magnetoresistance of investigated ceramic samples was measured as a function of temperature and magnetic field. Here MR was defined as MR (%)=[−(ρ H−ρ 0)/ρ 0]×100 %, where ρ 0 and ρ H are the resistivity in applied magnetic field of zero and 3 kOe, respectively. The temperature-dependent transportation of La2/3Ca1/3MnO3 is shown in Fig. 4. The transition temperature (T MI) was 265.08 K, and the peak MR was recorded up to 22.96 % at 257.51 K. The resistivity-versus-temperature and MR curves for LCSMO samples are shown clearly for different doping levels in Fig. 5 and Fig. 6. When Ca2+ was substituted by Sr2+, increase of T MI kept continuing as the doping level x of Sr increased. The changes of transition temperatures were shown clearly for different doping levels. T MI of samples with varied Sr concentrations all jumped above room temperature. The exact values are 289.71 K, 327.45 K, 352.05 K, and 393.23 K for the samples with x=0.2, 0.4, 0.6, and 1, respectively. Meanwhile, the peak MR values near T MI versus doping level different changed. The more Ca was substituted by Sr, the less peak MR amplitude became. For x=0.2, 0.4, and 0.6, the maximum MR values were 17.26 %, 9.05 %, and 5.42 % at 3 kOe, and the corresponding transition temperatures were 285.41 K, 317.84 K, and 342.90 K, respectively.
In Fig. 7, the peak MR values and peak MR temperatures versus the doping level x are shown. It was shown that Ca2+ substituted by Sr2+ has a great impact on transport properties of LCSMO. We can find out that the temperature at which peak MR occur is almost proportional to the amount of Sr doping level, while the peak MR is nearly inverse-proportional to x. So we can deduce that an MR of 13.5 % can be obtained at room temperature of 300 K with amount of Sr doping level for x=0.29. So La2/3(Ca1−x Sr x )1/3MnO3 with x=0.29 was successfully synthesized by the above same synthesis method, followed by studies on the RTMR behavior. The results have shown that T MI was 305.30 K, and the MR effect was recorded up to 12.9 % at 298.55 K.
These results show that we can balance the peak MR values and the peak MR temperature by controlling the doping level x of A site cation for Re1−x A x MnO3. Generally, the MR near T MI is closely related to the Double Exchange (DE) mechanism. With the same other conditions, small average A site ion radius leads to lower T C and larger MR near T MI. A site doping cations (A2+) do not participate in the DE interaction but show influence through coupling with B site (Mn) cations, and finally alter the Curie temperature (T C) and MR decreases. Considering a substitution of A site cations by ions with different lattice energy, it is expected that a ceramic with transition temperature and significant MR value in room temperature range and low magnetic field is possible. Since Sr2+ has a larger cation radius than that of Ca2+, when concentration of Sr2+ become larger, the bond angle and bond length of Mn–O–Mn pairs are changed and lead to T MI raised and peak MR value near T MI dropped. In our case, even when Ca2+ is gradually substituted by Sr2+, since both ions are of the same valence, the Mn3+/Mn4+ is not changed. But the radius of Sr2+ is larger than Ca2+, therefore the average A site cation radius is increased, and then T C (related to T MI) shifts to higher temperature while peak MR near T MI drops. We can obtain the significant MR value in room temperature range and low magnetic field to satisfy practical applications by controlling the doping level x of Sr for LCSMO.
On the other hand, the results also showed a jump at x=0.4 in Fig. 7. So we have further processed the XRD data in Fig. 2, the crystallite sizes were refined by Rietveld refinement technique, and the cell parameters of all the samples are listed in Table 1. It should be noticed the lattice parameters increase with Sr content. The LCSMO compounds have undergone a structural orthorhombic-to-monoclinic transition during the variation of x from 0.4 to 0.6. These results are similar to the observation obtained by Brankovic et al. [23], in which they have reported that with increasing values of dopant radius (Ca2+<Sr2+) transition temperature tend to be shifted to higher temperature and decreased the magnetoresistance. A systematic investigation by Thanh et al. [29, 30] for the ferromagnetic phase transitions and critical behavior of La0.7Ca0.3−x Sr x MnO3 (x=0, 0.05, 0.1, 0.2, and 0.25) single crystals also showed the structure changes during the variation of x, and it was at x=0.15. This can explain the reason of the jump at x=0.4 in Fig. 7.
4 Conclusions
Nanocrystalline La2/3(Ca1−x Sr x )1/3MnO3 materials were successfully synthesized by an improved high-energy ball milling with ethanol as a liquid medium and post sintering method. We have studied the microstructure, electrical transport and low-field MR effect by changing the value of x in La2/3(Ca1−x Sr x )1/3MnO3. The results showed that T MI increased with increasing doping level x. On the contrary, the peak MR values near T MI dropped with the more Ca2+ substituted. And we deduced that the low-field MR effect at room temperature could be obtained by controlling the doping Sr2+ substituted Ca2+ level. In La2/3(Ca1−x Sr x )1/3MnO3 with x=0.29, T MI was 305.30 K, and the MR effect was recorded up to 12.9 % at 298.55 K and 3 kOe.
References
Zener, C.: Phys. Rev. 82, 403 (1951)
Anderson, P.W., Hasegawa, H.: Phys. Rev. 100, 675 (1955)
Schiffer, P., Ramirez, A.P., Bao, W., Cheong, S.W.: Phys. Rev. Lett. 75, 3336 (1995)
Sun, J.R., Rao, G.H., Zhang, Y.Z.: Appl. Phys. Lett. 72, 3208 (1998)
Akther Hossain, A.K.M., Cohen, L.F., Kodenkandeth, T., MacManus-Driscoll, J., McN. Alford, N.: J. Magn. Magn. Mater. 195, 31 (1999)
Mori, S., Chen, C.H., Cheong, S.W.: Nature (London) 392, 473 (1998)
Millis, A.J., Littlewood, P.B., Shraiman, B.I.: Phys. Rev. Lett. 74, 5144 (1995)
Zheng, R.K., Li, G., Tang, A.N., Yang, Y., Wang, W., Li, X.G., Wang, Z.D., Ku, H.C.: Appl. Phys. Lett. 83, 5250 (2003)
Terai, T., Murata, T., Fukuda, T., Kakeshita, T.: J. Phys. Condens. Matter 16, 5801 (2004)
Gupta, A., Gong, G.Q., Xiao, G., Duncombe, P.R., Lecoeur, P., Trouilloud, P., Wang, Y.Y., Dravid, V.P., Sun, J.Z.: Phys. Rev. B 54, 629 (1996)
Hwang, H.Y., Cheong, S.W., Radaelli, P.G., Marezio, M., Batlogg, B.: Phys. Rev. Lett. 75, 914 (1995)
de Andres, A., Taboada, S., Colino, J.M., Ramirez, R., Garcia-Hernandez, M., Martinez, J.L.: Appl. Phys. Lett. 81, 319 (2002)
Shlyakhtin, O.A., Shin, K.H., Oh, Y.J.: J. Appl. Phys. 91, 7403 (2002)
Huang, B.X., Liu, Y.H., Zhang, R.Z., Xiao, B.Y., Wang, C.J., Mei, L.M.: J. Phys. D 36, 1923 (2003)
Wang, T., Chen, X.M., Wang, F.F., Shi, W.Z.: Physica B 405, 3088 (2010)
Gupta, A., Sun, J.Z.: J. Magn. Magn. Mater. 200, 24 (1999)
Li, X.W., Gupta, A., Xiao, G., Gong, G.Q.: Appl. Phys. Lett. 71, 1124 (1997)
Hwang, H.Y., Cheong, S.W., Ong, N.P., Batlogg, B.: Phys. Rev. Lett. 77, 2041 (1996)
Wang, Z.H., Ji, T.H., Wang, Y.Q., Chen, X., Li, R.W., Cai, J.W., Sun, J.R., Shen, B.G., Yan, C.H.: J. Appl. Phys. 87, 5582 (2000)
Siwach, P.K., Singh, H.K., Srivastava, O.N.: J. Phys. Condens. Matter 20, 273 (2008)
Gaur, A., Varma, G.D.: J. Alloys Compd. 453, 423 (2008)
Mikhaleva, E.V., Vasil’ev, V.G., Nosov, A.P., Vladimirova, E.V., Slobodin, B.V.: Phys. Chem. Glasses 35, 81 (2009)
Brankovic, Z., Đuris, K., Radojkovic, A., Bernik, S., Jaglicic, Z., Jagodic, M., Vojisavljevic, K., Brankovic, G.: J. Sol-Gel Sci. Technol. 55, 311 (2010)
Xiong, C.S., Mai, Y.T., Xiong, Y.H., Pi, H.L., Ren, Z.M., Zhang, J., Pi, Y.B., Xu, W., Dai, G.H., Song, S.J., Yang, S.: Mater. Res. Bull. 42, 904 (2007)
Jin, Z.Q., Tang, W., Zhang, J.R., Du, Y.W.: J. Magn. Magn. Mater. 187, 237 (1998)
Muroi, M., Street, R., McCormick, P.G.: J. Solid State Chem. 152, 503 (2000)
Muroi, M., Street, R., McCormick, P.G.: J. Appl. Phys. 87, 3424 (2000)
Suryanarayana, C.: Prog. Mater. Sci. 46, 1 (2001)
Thanh, T.D., Nguyen, L.H., Manh, D.H., Chien, N.V., Phong, P.T., Khiem, N.V., Hong, L.V., Phuc, N.X.: Physica B 407, 145 (2012)
Phan, M.H., Franco, V., Bingham, N.S., Srikanth, H., Hur, N.H., Yu, S.C.: J. Alloys Compd. 508, 238 (2010)
Acknowledgements
This work was supported by the National Science Foundation of China (Grant No. 20971048). The authors thank Analytical and Testing Center of Huazhong University of Science and Technology, which supplied us the facilities to fulfill the measurement.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Li, D.G., Mai, Y.T., Xoing, J. et al. Studies on Low-Field and Room-Temperature Magnetoresistance in La2/3(Ca1−x Sr x )1/3MnO3 Perovskites. J Supercond Nov Magn 26, 719–723 (2013). https://doi.org/10.1007/s10948-012-1798-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-012-1798-4