Abstract
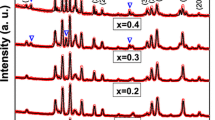
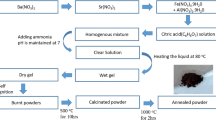
An investigation of the synthesis of BaFe12O19 powders by the organic acid precursor method is reported by acidic and neutral media. X-ray diffraction (XRD), scanning electron microscopy (SEM), and vibrating sample magnetometer (VSM) are utilized to study the effect of organic precursor type and annealing temperature on the crystal structure, crystallite size, microstructure and magnetic properties of the formed powders. The XRD analysis showed that the crystalline BaFe12O19 phase was obtained at 1200 °C for 2 h using different carboxylic acids in acidic medium. However, pure BaFe12O19 was achieved at low annealing temperature 1000 °C in neutral medium. SEM micrographs showed that the particles were strongly influenced by type of carboxylic acid and the annealing temperature. VSM study indicated that the saturation magnetization was increased with increasing annealing temperature to 1200 °C as the result of formation of single barium hexaferrites phase. High saturation magnetization (M s =66.5 emu/g) was achieved for the formed powders in neutral medium using tartaric acid as organic precursor. Wide coercivities of the formed powders (H c =259–5114 Oe) were obtained.





Similar content being viewed by others
References
Xu, G., Ma, H., Zhong, M., Zhou, J., Yue, Y., He, Z.: Influence of pH on characteristics of BaFe12O19 powder prepared by sol–gel auto-combustion. J. Magn. Magn. Mater. 301, 383–388 (2006)
Rashad, M.M., Radwan, M., Hessien, M.M.: Effect of Fe/Ba mole ratios and surface-active agents on the formation and magnetic properties of co-precipitated barium hexaferrite. J. Alloys Compd. 453, 304–309 (2008)
Hessien, M.M., Radwan, M., Rashad, M.M.: Enhancement of magnetic properties for the barium hexaferrite prepared through ceramic route. J. Anal. Appl. Pyrolysis 78, 282–287 (2007)
Radwan, M., Rashad, M.M., Hessien, M.M.: Synthesis and characterization of barium hexaferrite nanoparticles. J. Mater. Process. Technol. 181, 106–109 (2007)
Rashad, M.M., Ibrahim, I.A.: Improvement of the magnetic properties of barium hexaferrite nanopowders using modified co-precipitation method. J. Magn. Magn. Mater. 323, 2158–2162 (2011)
Yu, H.-F., Liu, P.-C.: Effects of pH and calcination temperatures on the formation of citrate-derived hexagonal barium ferrite particles. J. Alloys Compd. 416, 222–227 (2006)
Mohamed, R.M., Rashad, M.M., Haraz, F.A., Sigmund, W.: Structure and magnetic properties of nanocrystalline cobalt ferrite powders synthesized using organic acid precursor method. J. Magn. Magn. Mater. 322, 2058–2064 (2010)
Rashad, M.M.: Effect of synthesis conditions on the preparation of bismuth ferrite nanopowders via two different routes. J. Mater. Sci., Mater. Electron. 23, 882–888 (2012)
Mohsen, Q.: Barium hexaferrite synthesis by oxalate precursor route. J. Alloys Compd. 500, 125–128 (2010)
Junliang, L., Wei, Z., Cuijing, G., Yanwei, Z.: Synthesis and magnetic properties of quasi-single domain M-type barium hexaferrite powders via sol–gel auto-combustion: effects of pH and the ratio of citric acid to metal ions (CA/M). J. Alloys Compd. 479, 863–869 (2009)
Mali, A., Ataie, A.: Influence of the metal nitrates to citric acid molar ratio on the combustion process and phase constitution of barium hexaferrite particles prepared by sol–gel combustion method. Ceram. Int. 30, 1979–1983 (2004)
Hessien, M.M., Rashad, M.M., El-Barawy, K.: Controlling the composition and magnetic properties of strontium hexaferrite synthesized by co-precipitation method. J. Magn. Magn. Mater. 320, 336–343 (2008)
Acknowledgement
This research is financially supported by the Science and Technology Development Fund (STDF), Egypt, Grant No. Project ID 246.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rashad, M.M., Ibarhim, I.A. Synthesis and Magnetic Properties of Barium Hexaferrite Powders Using Organic Acid Precursor Method. J Supercond Nov Magn 26, 1639–1644 (2013). https://doi.org/10.1007/s10948-012-1871-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-012-1871-z