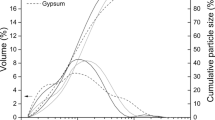
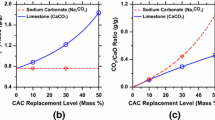
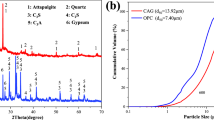
Abstract
The low temperature of decomposition of some calcium carbonates and the bending of the TG curves of hydrated cement between 500 and 800°C suggested the presence of some complex compound(s), which needed complementary investigation (XRD, TG). Stepwise transformation of portlandite (and/or lime) into calcium carbonate, with intermediate steps of calcium carbonate hydroxide hydrates (CCH-1 to CCH-5), was indicated by the previous study of two OPC.
This was checked here on four cements ground for t g=15, 20, 25 and 30 min and hydrated either in water vapour, successively at RH=1.0, 0.95 and 0.5 for 2 weeks each (WR1, WR2 and WR3, respectively) or as mortars in liquid water (1m), followed by WR as above. The d[001] spacing of portlandite was confirmed to vary: here between the lowest and the highest standard values. The diffractograms of n=32 different samples were analyzed for presence of standard CCH peaks, generally slightly displaced. These were: CCH-1 [Ca3(CO3)2(OH)2]: N=11 peaks, of three different d[hkl] spacings, CCH-2 [Ca6(CO2.65)2(OH657)7(H2O)2]: N=10 for two d[hkl], CCH-3 [Ca3(CO3)2(OH)2·1.5H2O]: N=14 for five d[hkl], CCH-4, ikaite [CaCO3(H2O)6]: N=13 for six d[hkl], CCH-5[CaCO3(H2O)]: N=15 for five d[hkl]. Thus the most probable is the presence of the last three. The stepwise transformation of Ca(OH)2 into CaCO3 was confirmed:
portlandite (varying d[001])→CCH-1→CCH-2→CCH-3→CCH-4→CCH-5→CaCO3
The content of CCH was the highest at t gr=15 min, decreasing down to t gr=25 min and increasing slightly at 30 min, as inferred from the number of the peaks observed. After cement powder hydration at RH=1.0 (WR1) peak number increased gradually from CCH-1 to CCH-5, whereas in the hydrated mortar (1m) the peak number decreased from CCH-1 to CCH-5, indicating the respective progress of the carbonation reaction.
Similar content being viewed by others
References
SC Mojumdar K Mazanec M Drabik (2006) J. Therm. Anal. Cal. 83 135 Occurrence Handle10.1007/s10973-005-7045-5 Occurrence Handle1:CAS:528:DC%2BD28XitF2ntrk%3D
C Tomasi O Ricci G Perotti P Ferloni (2006) J. Therm. Anal. Cal. 84 33 Occurrence Handle10.1007/s10973-005-7264-9 Occurrence Handle1:CAS:528:DC%2BD28XjsVOmsb0%3D
RMH Lawrence TJ Mays P Walker D D’Ayala (2006) J. Therm. Anal. Cal. 85 377 Occurrence Handle10.1007/s10973-005-7302-7 Occurrence Handle1:CAS:528:DC%2BD28Xptlagsbc%3D
I Janotka SC Mojumdar (2005) J. Therm. Anal. Cal. 81 197 Occurrence Handle10.1007/s10973-005-0767-6 Occurrence Handle1:CAS:528:DC%2BD2MXlslOltrk%3D
ET Stepkowska JM Martinez-Blanes A Justo MA Aviles JL Perez-Rodriguez (2005) J. Therm. Anal. Cal. 80 193 Occurrence Handle10.1007/s10973-005-0635-4 Occurrence Handle1:CAS:528:DC%2BD2MXktl2ksro%3D
ET Stepkowska (2005) J. Therm. Anal. Cal. 80 727 Occurrence Handle10.1007/s10973-005-0721-7 Occurrence Handle1:CAS:528:DC%2BD2MXks1CjtLw%3D
ET Stepkowska (2006) J. Therm. Anal. Cal. 84 1 Occurrence Handle10.1007/s10973-005-7179-5 Occurrence Handle1:CAS:528:DC%2BD28XjsVOntbc%3D
ET Stepkowska JM Bijen JL Perez-Rodriguez A Justo PJ Sanchez-Soto MA Aviles (1994) J. Thermal Anal. 42 41 Occurrence Handle10.1007/BF02546991 Occurrence Handle1:CAS:528:DyaK2cXmtFWmsro%3D
G Schimmel (1970) Natura 57 38 Occurrence Handle1:CAS:528:DyaE3cXosFKqtQ%3D%3D
G Schimmel (1970) Phys. Blatter 5 213
BW Liebich H Sarp (1985) Schweiz. Mineral. Petrogr. Mitt. 65 153 Occurrence Handle1:CAS:528:DyaL28XmtFOjsr8%3D
D. Smith and Schultz, Penn State University, University Park, Pennsylvania, USA, ICD Grant-in-Aid (1983).
A. Winchell and H. Winchell, Microscopic Character of Artificial Inorg. Solid Sub. 95, 1964.
B Dickens WE Brown (1970) Inorg. Chem. 9 480 Occurrence Handle10.1021/ic50085a010 Occurrence Handle1:CAS:528:DyaE3cXpsVWqug%3D%3D
KF Hesse H Kueppers E Suess (1983) Z. Kristallogr. 163 227 Occurrence Handle1:CAS:528:DyaL3sXlsl2itLo%3D Occurrence Handle10.1524/zkri.1983.163.3-4.227
G Taylor (1975) Am. Mineral. 60 690 Occurrence Handle1:CAS:528:DyaE2MXls1Kqtbw%3D
H Effenberger (1981) Monatsh. Chem. 112 899 Occurrence Handle10.1007/BF00905061 Occurrence Handle1:CAS:528:DyaL3MXls1Sgtr4%3D
H Effenberger K Lueger-Ging (1980) European Crystallographic Meeting 6 107
ET Stepkowska JL Perez-Rodriguez MJ Sayagues JM Martinez-Blanes (2003) J.Therm. Anal. Cal. 73 247 Occurrence Handle10.1023/A:1025158213560 Occurrence Handle1:CAS:528:DC%2BD3sXmt1emt70%3D
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stepkowska, E.T., Aviles, M.A., Blanes, J.M. et al. Gradual transformation of Ca(OH)2 into CaCO3 on cement hydration. J Therm Anal Calorim 87, 189–198 (2007). https://doi.org/10.1007/s10973-006-7840-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-006-7840-7