Abstract
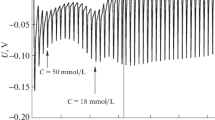
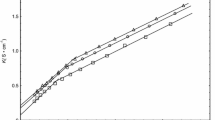
The molality dependence of specific conductivity of pentadecyl bromide, cetylpyridinium bromide and cetylpiridinium chloride in aqueous solutions has been studied in the temperature range of 30–45 °C. The critical micelle concentration (cmc) and ionization degree of the micelles, β, were determined directly from the experimental data. Thermal parameters, such as standard Gibbs free energy \( \Updelta G_{m}^{0} , \) enthalpy \( \Updelta H_{m}^{0} \) and entropy \( \Updelta S_{m}^{0} , \) of micellization were estimated by assuming that the system conforms to the pseudo-phase separation model. The change in heat capacity on micellization \( \Updelta C_{p} , \) was estimated from the temperature dependence of \( \Updelta H_{m}^{0} . \) An enthalpy–entropy compensation phenomenon for the studied system has been found.









Similar content being viewed by others
References
Jönson B, Lindman B, Holmberg K, Kronberg B. Surfactants and polymers in aqueous solution. 1st ed. Chichester: John Wiley and Sons; 1998.
Galán JJ, González-Pérez A, Del Castillo JL, Rodríguez JR. Thermal parameters associated to micellization of dodecylpyridinium bromide and chloride in aqueous solution. J Therm Anal Calorim. 2002;70:229–34.
Galán JJ, González-Pérez A, Rodríguez JR. Micellization of dodecyldimethylethyl-ammonium bromide in aqueous solution: thermal parameters. J Therm Anal Calorim. 2003;72:465–70.
Myers D. Surfactant science and technology. 2nd ed. New York: VHC Publishers; 1992.
Starks CM, Liotta CL, Halpern MH. Phase transfer catalysis: fundamentals, applications and industrial perspectives. 1st ed. London: Chapman and Hall; 1994.
Goworek J, Agnieszka K, Gac W, Borówka A, Kusak R. Thermal degradation of CTAB in AS-synthesized MCM-41. Therm Anal Calorim. 2009;96(2):375–82.
Liu R, Yang J, Sun C, Wu X, Li L, Su B. Study on the interaction between nucleic acids and cationic surfactants. Colloid Surf B. 2004;34:59–63.
Kozak M, Domka L, Jurga S. Interactions of cationic surfactants with DPPC. J Therm Anal Calorim. 2007;88(2):395–9.
Trikalitis PN, Rangan KK, Bakas T, Kanatzidis MG. Single-crystal mesostructured semiconductors with cubic Ia3d symmetry and ion-exchange properties. J Am Chem Soc. 2002;124(41):12255–60.
Eisenschmidt K, Lanio T, Simoncsits A, Jeltsch A, Pingoud V, Wende W, et al. Developing a programmed restriction endonuclease for highly specific DNA cleavage. Nucleic Acids Res. 2005;33:7039–47.
Mishraa A, Beheraa RK, Mishrab BK, Beherab GB. Dye–surfactant interaction: chain folding during solubilization of styryl pyridinium dyes in sodium dodecyl sulfate aggregates. J Photochem Photobiol A. 1999;121(1):63–73.
Ghosh KK, Roy S. Thermodynamics of micelle formation of some cationic surfactants as a function of temperature and solvent. Indian J Chem B. 1998;37(9):875–80.
Monk CB. Electrolytic dissociation. 1st ed. London: Academic Press; 1961.
Skerjanc J, Kogej K, Cerar J. Equilibrium and transport properties of alkylpyridinium bromides. Langmuir. 1999;15:5023–8.
Fujio K, Mitsui T, Kurumizawa H, Tanaka Y, Uzu Y. Solubilization of water-insoluble dye in aqueous solutions of alkylpyridinium bromides and its relation to micellar size and shape. Colloid Polym Sci. 2004;282:223–9.
Gharibi H, Palepu R, Bloor DM, Hall DG, Wyn-Jones E. Electrochemical studies associated with micellization of cationic surfactants in ethylene glycol. Langmuir. 1992;8:782–7.
Malsch J, Hartley G. The wien effect of a long-chain salt in aqueous solutions and for short impact duration of pratical alterations of measurement. Z Phys Chem. 1934;170:321–36.
Molinero I, Sierra ML, Valientes M, Rodenas E. Physical properties of cetylpyridinium chloride micelles and their behaviour as reaction media. J Chem Soc Faraday Trans. 1996;92:59–63.
Chen LJ, Lin SY, Huang CC. Effect of hydrophobic chain of surfactants on enthalpy–entropy compensation of micellization. J Phys Chem B. 1998;102:4350–6.
Galán JJ, González-Pérez A, Seijas JA, Uriarte E, Rodríguez JR. Effect of counterion on thermodynamic micellar properties of tetradecylpyridinium in aqueous solution. Colloid Polym Sci. 2005;283:456–60.
Zielinsky R, Ikeda S, Nomura H, Kato S. Adiabatic compressibility of alkyltrimethylammonium bromides in aqueous solution. J Colloid Interface Sci. 1987;119:398–408.
González-Pérez A, Czapkiewicz J, Del Castillo JL, Rodríguez JR. Micellar behaviour of tetradecyldimethylbenzylammonium chloride in water-alcohol mixtures. J Colloid Interface Sci. 2003;262(2):525–30.
Moroi Y. Micelles: theoretical and applied aspects. New York: Plenum Press; 1992.
Mehrian T, Keizer A, Korteweg AJ, Lyklema J. Thermodynamics of micellization of n-alkylpyridinium chlorides. Colloids Surf A. 1993;71:255–67.
Muller N. Temperature dependence of critical micelle concentrations and heat capacities of micellization for ionic surfactants. Lagmuir. 1993;9(1):96–100.
Sugihara G, Hisatomi M. Entalpy–entropy compensation phenomenon observed for different surfactants in aqueous solution. J Colloid Interface Sci. 1999;219:31–6.
Tartar HV. CMC of aqueous solutions of paraffin chain salts regarded as a function of thickness of ionic atmosphere. J Colloid Sci. 1962;17(3):243–8.
Acknowledgements
The authors wish to express their thanks for the financial support of the Dirección Xeral de I + D+I of the Xunta de Galicia and the European Regional Development Fund (INCITE07PXI206076ES).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Galán, J.J., Rodríguez, J.R. Thermodynamic study of the process of micellization of long chain alkyl pyridinium salts in aqueous solution. J Therm Anal Calorim 101, 359–364 (2010). https://doi.org/10.1007/s10973-009-0385-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-009-0385-9