Abstract
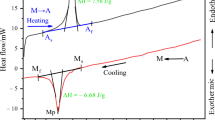
In the present study, the transformation temperatures and shape memory effect of the Cu-14.82 wt% Al-0.4 wt% Be shape memory alloy were studied. The structural properties of the alloys were characterized by X-ray diffraction. The crystal structure analysis of the alloy shows a thermoelastic transformation from an ordered parent-phase of DO3 type to the M18R martensite. The microstructure of the alloy was investigated by optical micrographs. The transformation temperatures, enthalpy and entropy values of the alloy were determined by differential scanning calorimetry. Thermogravimetric and differential thermal analysis measurements were performed to obtain the ordered–disordered phase transformations. The activation energy values determined from two different methods were found to be 306.53 kJ mol−1 for Kissinger and 298.57 kJ mol−1 Ozawa.







Similar content being viewed by others
References
Mallik U, Sampath V. Influence of aluminum and manganese concentration on the shape memory characteristics of Cu–Al–Mn shape memory alloys. J Alloy Compd. 2008;459:142–7.
Hsu CA, Wang WH, Hsu YF, Rehbach WP. The refinement treatment of martensite in Cu-11.38 wt% Al-043 wt% Be shape memory alloys. J Alloy Compd. 2009;474:455–62.
Montecinos S, Cuniberti A, Sepulveda A. Grain size and pseudoelastic behaviour of a Cu–Al–Be alloy. Mater Charact. 2008;59:117–23.
Silva RAG, Machado ES, Adorno AT, Magdelena AG, Carvalho TM. Completeness of β-phase decomposition reaction in Cu–Al–Ag alloys. J Therm Anal Calorim. 2012;109:927–31.
Kainuma R, Satoh N, Liu XJ, Ohnuma I, Ishida K. Phase equilibria and Heusler phase stability in the cu-rich portion of the Cu–Al–Mn system. J Alloy Compd. 1998;266(1–2):191–200.
Liu XJ, Ohnuma I, Kainuma R, Ishida K. Phase equiliria in the cu-rich portion of the Cu–Al binary system. J Alloy Compd. 1998;264:201–8.
Magdelena AG, Adorno AT, Silva RAG, Carvalho TM. Effect of Ag concentration on the thermal behavior of the Cu-10 mass % Al and Cu-11 mass % Al alloys. J Therm Anal Calorim. 2009;97(1):47–51.
Montecinos S, Cuniberti A, Castro ML, Boeri R. Phase transformations during continuous cooling of polycrystalline β-CuAlBe alloys. J Alloy Compd. 2009;467(1–2):278–83.
Montecinos S, Cuniberti A. Thermomechanical behavior of a CuAlBe shape memory alloy. J Alloy Compd. 2008;457:332–6.
Castro ML, Romero R. Isothermal decomposition of the Cu-22.72 Al-3.55 Be at.% alloy. Mat Sci Eng-A Struct. 2000;A287:66–71.
Montecinos S, Cuniberti A, Castro ML. Kinetics of isothermal decomposition in polycrystalline β CuAlBe alloys. Intermetallics. 2010;18(1):36–41.
Silva RAG, Adorno AT, Magdelena AG, Carvalho TM, Stipcich M, Cuniberti A, Castro ML. Thermal behavior of the Cu-22.55 at.% Al alloy with small Ag additions. J Therm Anal Calorim. 2011;103:459–63.
Marcos J, Manso L, Planes A, Romero R, Castro ML. Kinetics of the phase separation in Cu–Al–Mn alloys and the ınfluence on martensitic transformations. Philos Mag. 2004;84(1):45–68.
Aksu Canbay C. The production of Cu-based shape memory alloys and ınvestigation of microstructural, thermal and electrical properties of alloys, Ph.D Thesis. Fırat University, Institue of Science, Elazığ/Turkey (Turkish) 2010.
Chentouf SM, Bouabdallah M, Cheniti H, Eberhardt A, Patoor E, Sari A. Ageing study of Cu–Al–Be hypoeutectoid shape memory alloy. Mater Charact. 2010;61:1187–93.
Lu X, Chen F, Li W, Zheng Y. Effect of Ce addition on the microstructure and damping properties of Cu–Al–Mn shape memory alloys. J Alloy Compd. 2009;480:608–11.
Suresh N, Ramamurty U. Effect of aging on mechanical behavior of single crystal Cu–Al–Ni shape memory alloys. Mat Sci Eng-A Struct. 2007;A454–455:492–9.
Suresh N, Ramamurty U. Aging response and its effect on functional properties of Cu–Al–Ni shape memory alloys. J Alloy Compd. 2008;499:113–8.
Perez-Landazabal JI, Recarte V, Sanchez-Alarcos V, No ML, San Juan J. Study of stability and decomposition of the β phase in Cu–Al–Ni shape memory alloy. Mat Sci Eng-A Struct. 2006;438–440:734–7.
Meng Q, Yang H, Liu Y, Nam T. Transformation ıntervals and elastic strain energies of B2–B19 martensitic transformation of NiTi. Intermetallics. 2010;18:2431–4.
Prado MO, Decorte PM, Lovey F. Martensitic transformation in Cu–Mn–Al alloys. Scripta Metall Mater. 1995;33(6):878–83.
Salzbrenner RJ, Cohen M. On the thermodynamics of thermoelastic martensitic transformations. Acta Metall. 1979;27(5):739–48.
Kato H, Yasuda Y, Sasaki K. Thermodynamic assessment of the stabilization effect in deformed shape memory alloy martensite. Acta Metall. 2011;59:3955–64.
Ortin J, Planes A. Thermodynamic analysis of thermal measurements in thermoelastic martensitic transformations. Acta Metall. 1988;37:1873–89.
Obrado E, Manosa L, Planes A. Influence of composition and thermal treatments on the martensitic transition of Cu–Al–Mn alloys. J Phys IV. France 7 (1997) Colloque C5, Supplement au Jour. De Phys. III de Novembre.
Vyazovkin S, Burnham AK, Criado JM, Perez-Maqueda LA, Popescu C, Sbirrazzuoli N. ICTAC kinetics committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta. 2011;20:1–19.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29(11):1702–6.
Ozawa T. Kinetic analysis of derivative curves in thermal analysis. Anal Calorim. 1970;2(3):321–4.
Cuniberti A, Montecinos S, Lovey FC. Effect of gamma(2)-phase precipitates on the martensitic transformation of a β-CuAlBe shape memory alloy. Intermetallics. 2009;17(6):435–40.
Aksu Canbay C, Aydoğdu A. Thermal and microstructural properties of Cu–Al–Be shape memory alloys, Turkish Physical Society 27th International Physics Congress, İstanbul-Türkiye, Eylül 2010.
Acknowledgements
This study is financially supported by TÜBİTAK, Project No.: 106T583.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aksu Canbay, C., Aydoğdu, A. Thermal analysis of Cu-14.82 wt% Al-0.4 wt% Be shape memory alloy. J Therm Anal Calorim 113, 731–737 (2013). https://doi.org/10.1007/s10973-012-2792-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-012-2792-6