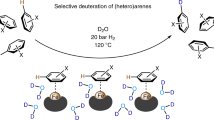
Abstract
A simple and efficient flow-based technique is reported for the catalytic deuteration of several model nitrogen-containing heterocyclic compounds which are important building blocks of pharmacologically active materials. A continuous flow reactor was used in combination with on-demand pressure-controlled electrolytic D2 production. The D2 source was D2O, the consumption of which was very low. The experimental set-up allows the fine-tuning of pressure, temperature, and flow rate so as to determine the optimal conditions for the deuteration reactions. The described procedure lacks most of the drawbacks of the conventional batch deuteration techniques, and additionally is highly selective and reproducible.
Similar content being viewed by others
References
Blake MI, Crespi HL, Katz J (1975) Studies with deuterated drugs. J Pharm Sci 64: 367–391. doi:10.1002/jps.2600640306
Baldwin JE, Raghavan AS, Hess BA, Smentek L (2006) Thermal [1,5] hydrogen sigmatropic shifts in cis,cis-1,3-cyclononadienes probed by gas-phase kinetic studies and density functional theory calculations. J Am Chem Soc 128: 14854–14862. doi:10.1021/ja065656s
Kharasch ED, Bedynek PS, Park S, Whittington D, Walker A, Hoffer C (2008) Mechanism of ritonavir changes in methadone pharmacokinetics and pharmacodynamics: I. Evidence against CYP3A mediation of methadone clearance. Clin Pharmacol Ther 84: 497–505. doi:10.1038/clpt.2008.104
Perrin CL, Lau JS (2006) Hydrogen-bond symmetry in zwitterionic phthalate anions: symmetry breaking by solvation. J Am Chem Soc 128: 11820–11824. doi:10.1021/ja063797o
Salzmann M, Pervushin K, Wider G, Senn H, Wüthrich K (1998) TROSY in triple-resonance experiments: new perspectives for sequential NMR assignment of large proteins. Proc Natl Acad Sci USA 95: 13585–13590
Zhou H, Ranish JA, Watts JD, Aebersold R (2002) Quantitative proteome analysis by solid-phase isotope tagging and mass spectrometry. Nat Biotechnol 20: 512–515. doi:10.1038/nbt0502-512
Skaddan MB, Yung CM, Bergman RG (2004) Stoichiometric and catalytic deuterium and tritium labeling of “unactivated” organic substrates with cationic Ir(III) complexes. Org Lett 6: 11–13. doi:10.1021/ol0359923
Kurita T, Aoki F, Mizumoto T, Maejima T, Esaki H, Maegawa T, Monguchi Y, Sajiki H (2008) Facile and convenient method of deuterium gas generation using a Pd/C-catalyzed H-2-D-2 exchange reaction and its application to synthesis of deuterium-labelled compounds. Chem Eur J 14: 3371–3379. doi:10.1002/chem.200701245
Maegawa T, Fujiwara Y, Inagaki Y, Esaki H, Monguchi Y, Sajiki H (2008) Mild and efficient H/D exchange of alkanes based on C–H activation catalyzed by rhodium on charcoal. Angew Chem Int Ed 47: 5394–5397. doi:10.1002/anie.200800941
Darvas F, Dorman G, Lengyel L, Kovacs I, Jones R, Urge L (2009) High pressure, high temperature reactions in continuous flow merging discovery and process chemistry. Chim Oggi 27: 40–43
Belfiore LA (2003) Transport phenomena for chemical reactor design. Wiley, New Jersey
Jones RV, Godorhazy L,Varga N, Szalay D, Urge L, Darvas F (2006) Continuous-flow high pressure hydrogenation reactor for optimization and high-throughput synthesis. J Comb Chem 8:110–116. doi:10.1021/cc050107o; http://www.thalesnano.com
Mandity IM, Martinek TA, Darvas F, Fülöp F (2009) A simple, efficient, and selective deuteration via a flow chemistry approach. Tetrahedron Lett 50: 4372–4374. doi:10.1016/j.tetlet.2009.05.050
Brase S, Gil C, Knepper K (2002) The recent impact of solid-phase synthesis on medicinally relevant benzoannelated nitrogen heterocycles. Bioorg Med Chem 10: 2415–2437. doi:10.1016/S0968-0896(02)00025-1
Noble S, Faulds D (1996) Saquinavir—a review of its pharmacology and clinical potential in the management of HIV infection. Drugs 52: 93–112
Palmer KJ, Holliday SM, Brogden RN (1993) Mefloquine—a review of its antimalarial activity, pharmacokinetic properties and therapeutic efficacy. Drugs 45: 430–475
Houlihan WJ, Munder PG, Handley DA, Cheon SH, Parrino VA (1995) Antitumor activity of 5-aryl-2,3-dihydroimidazo[2,1-A]isoquinolines. J Med Chem 38: 234–240. doi:10.1021/jm00002a004
Brogden RN, Heel RC, Speight TM, Avery GS (1979) Nomifensine—review of its pharmacological properties and therapeutic efficacy in depressive-illness. Drugs 18: 1–24
Jarvis B, Markham A (2000) Montelukast—a review of its therapeutic potential in persistent asthma. Drugs 59: 891–928
Davis R, Markham A, Balfour JA (1996) Ciprofloxacin—an updated review of its pharmacology, therapeutic efficacy and tolerability. Drugs 51: 1019–1074
Hazeldine ST, Polin L, Kushner J, Paluch J, White K, Edelstein M, Palomino E, Corbett TH, Horwitz JP (2001) Design, synthesis, and biological evaluation of analogues of the antitumor agent, 2-{4-[(7-chloro-2-quinoxalinyl)oxy]phenoxy}-propionic acid (XK469). J Med Chem 44: 1758–1776. doi:10.1021/jm0005149
Cerecetto H, Di Maio R, Gonzalez M, Risso M, Saenz P, Seoane G, Denicola A, Peluffo G, Quijano C, Olea-Azar C (1999) 1,2,5-oxadiazole N-oxide derivatives and related compounds as potential antitrypanosomal drugs: structure–activity relationships. J Med Chem 42: 1941–1950. doi:10.1016/S0223-5234(01)01265-X
Kim KS, Qian L, Bird JE, Dickinson KE, Moreland S, Schaeffer TR, Waldron TL, Delaney CL, Weller HN, Miller AV (1993) Quinoxaline N-oxide containing potent angiotensin-II receptor antagonists—synthesis, biological properties, and structure–activity-relationships. J Med Chem 36: 2335–2342. doi:10.1021/jm00068a010
Sarges R, Howard HR, Browne RG, Lebel LA, Seymour PA, Koe BK (1990) 4-amino[1,2,4]triazolo[4,3-A]quinoxalines—A novel class of potent adenosine receptor antagonists and potential rapid-onset antidepressants. J Med Chem 33: 2240–2252. doi:10.1021/jm00170a031
Bentley KW (2006) Beta-Phenylethylamines and the isoquinoline alkaloids. Nat Prod Rep 23: 444–463. doi:10.1039/b509523a
Kushner DJ, Baker A, Dunstall TG (1999) Pharmacological uses and perspectives of heavy water and deuterated compounds. Can J Physiol Pharmacol 77: 79–88. doi:10.1139/cjpp-77-2-79
Derdau V (2004) Deuterated ammonium formate as deuterium source in a mild catalytic deuterium transfer reaction of pyridines, pyrazines and isoquinolines. Tetrahedron Lett 45: 8889–8893. doi:10.1016/j.tetlet.2004.09.165
Esaki H, Ito N, Sakai S, Maegawa T, Monguchi Y, Sajiki H (2006) General method of obtaining deuterium-labelled heterocyclic compounds using neutral D2O with heterogeneous Pd/C. Tetrahedron 62: 10954–10961. doi:10.1016/j.tet.2006.08.088
Sullivan JA, Flanagan KA, Hain H (2008) Selective H–D exchange catalysed by aqueous phase and immobilised Pd nanoparticles. Catal Today 139: 154–160. doi:10.1016/j.cattod.2008.03.031
Oba M, Terauchi T, Hashimoto J, Tanaka T, Nishiyama K (1997) Stereoselective synthesis of (2S,3S,4R,5S)-proline-3,4,5-d(3). Tetrahedron Lett 38: 5515–5518. doi:10.1016/S0040-4039(97)01189-1
Oba M, Ohkuma K, Hitokawa H, Shirai A, Nishiyama K (2006) Convenient synthesis of deuterated glutamic acid, proline and leucine via catalytic deuteration of unsaturated pyroglutamate derivatives. J Label Compd Radiopharm 49: 229–235. doi:10.1002/jlcr.1038
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ötvös, S.B., Mándity, I.M. & Fülöp, F. Highly selective deuteration of pharmaceutically relevant nitrogen-containing heterocycles: a flow chemistry approach. Mol Divers 15, 605–611 (2011). https://doi.org/10.1007/s11030-010-9276-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-010-9276-z