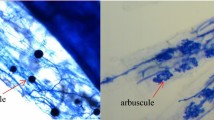
Abstract
Background and Aims
Plants growing on serpentine bedrock have to cope with the unique soil chemistry and often also low water-holding capacity. As plant-soil interactions are substantially modified by arbuscular mycorrhizal (AM) symbiosis, we hypothesise that drought tolerance of serpentine plants is enhanced by AM fungi (AMF).
Methods
We conducted a pot experiment combining four levels of drought stress and three AMF inoculation treatments, using serpentine Knautia arvensis (Dipsacaceae) plants as a model.
Results
AMF inoculation improved plant growth and increased phosphorus uptake. The diminishing water supply caused a gradual decrease in plant growth, accompanied by increasing concentrations of drought stress markers (proline, abscisic acid) in root tissues. Mycorrhizal growth dependence and phosphorus uptake benefit increased with drought intensity, and the alleviating effect of AMF on plant drought stress was also indicated by lower proline accumulation.
Conclusions
We documented the role of AM symbiosis in plant drought tolerance under serpentine conditions. However, the potential of AMF to alleviate drought stress was limited beyond a certain threshold, as indicated by a steep decline in mycorrhizal growth dependence and phosphorus uptake benefit and a concomitant rise in proline concentrations in the roots of mycorrhizal plants at the highest drought intensity.




Similar content being viewed by others
References
Al-Karaki G, McMichael B, Zak J (2004) Field response of wheat to arbuscular mycorrhizal fungi and drought stress. Mycorrhiza 14:263–269
Anacker B, Rajakaruna N, Ackerly D, Harrison S, Keeley J, Vasey M (2011) Ecological strategies in California chaparral: interacting effects of soils, climate, and fire on specific leaf area. Plant Ecol Divers 4:179–188
Aroca R, Bago A, Sutka M, Paz JA, Cano C, Amodeo G, Ruiz-Lozano JM (2009) Expression analysis of the first arbuscular mycorrhizal fungi aquaporin described reveals concerted gene expression between salt-stressed and nonstressed mycelium. Mol Plant Microbe Interact 22:1169–1178
Asch F (2000) Determination of abscisic acid by indirect enzyme linked immuno sorbent assay (ELISA). Technical report. Laboratory for Agrohydrology and Bioclimatology, Department of Agricultural Sciences, The Royal Veterinary and Agricultural University, Taastrup
Augé RM (2001) Water relations, drought and vesicular-arbuscular mycorrhizal symbiosis. Mycorrhiza 11:3–42
Augé RM (2004) Arbuscular mycorrhizae and soil/plant water relations. Can J Soil Sci 84:373–381
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Bolandnazar S, Aliasgarzad N, Neishabury MR, Chaparzadeh N (2007) Mycorrhizal colonization improves onion (Allium cepa L.) yield and water use efficiency under water deficit condition. Sci Hortic 114:11–15
Brady KU, Kruckeberg AR, Bradshaw HD (2005) Evolutionary ecology of plant adaptation to serpentine soils. Annu Rev Ecol Evol S 36:243–266
Caravaca F, Alguacil MM, Hernández JA, Roldán A (2005) Involvement of antioxidant enzyme and nitrate reductase activities during drought stress and recovery of mycorrhizal Myrtus communis and Phillyrea angustifolia plants. Plant Sci 169:191–197
Coleman R, Jove C (1992) Geological origin of serpentinites. In: Baker AJM, Proctor J, Reeves RD (eds) The vegetation of ultramafic (serpentine) soils. Intercept Ltd, Andover, pp 1–18
Danneberg G, Latus Z, Zimmer W, Hundeshagen B, Schneiderpoetsch H, Bothe H (1993) Influence of vesicular-arbuscular mycorrhiza on phytohormone balances in maize (Zea mays L.). J Plant Physiol 141:33–39
Davies FT, Olalde-Portugal V, Aguilera-Gomez L, Alvarado MJ, Ferrera-Cerrato RC, Boutton TW (2002) Alleviation of drought stress of Chile ancho pepper (Capsicum annuum L. cv. San Luis) with arbuscular mycorrhiza indigenous to Mexico. Sci Hortic 92:347–359
Davoodian N, Bosworth J, Rajakaruna N (2012) Mycorrhizal colonization of Hypericum perforatum L. (Hypericaceae) from serpentine and granite outcrops on the Deer Isles, Maine. Northeast Nat 19:517–526
Doubková P, Suda J, Sudová R (2011) Arbuscular mycorrhizal symbiosis on serpentine soils: the effect of native fungal communities on different Knautia arvensis ecotypes. Plant Soil 345:325–338
Doubková P, Suda J, Sudová R (2012) The symbiosis with arbuscular mycorrhizal fungi contributes to plant tolerance to serpentine edaphic stress. Soil Biol Biochem 44:56–64
Duan XG, Neuman DS, Reiber JM, Green CD, Saxton AM, Augé RM (1996) Mycorrhizal influence on hydraulic and hormonal factors implicated in the control of stomatal conductance during drought. J Exp Bot 47:1541–1550
Entry JA, Rygiewicz PT, Watrud LS, Donnelly PK (2002) Influence of adverse soil conditions on the formation and function of arbuscular mycorrhizas. Adv Environ Res 7:123–138
Fan QJ, Liu JH (2011) Colonization with arbuscular mycorrhizal fungus affects growth, drought tolerance and expression of stress-responsive genes in Poncirus trifoliata. Acta Physiol Plant 33:1533–1542
Fitzsimons MS, Miller RM (2010) Serpentine soil has little influence on the root-associated microbial community composition of the serpentine tolerant grass species Avenula sulcata. Plant Soil 330:393–405
Gerdemann JW, Nicolson TH (1962) Endogone spores in cultivated soils. Nature 195:308–309
Goicoechea N, Antolín MC, Sánchez-Díaz M (1997) Gas exchange is related to the hormone balance in mycorrhizal or nitrogen-fixing alfalfa subjected to drought. Physiol Plantarum 100:989–997
Huang Z, Zou ZR, He CX, He ZQ, Zhang ZB, Li JM (2011) Physiological and photosynthetic responses of melon (Cucumis melo L.) seedlings to three Glomus species under water deficit. Plant Soil 339:391–399
Hughes R, Bachmann K, Smirnoff N, Macnair MR (2001) The role of drought tolerance in serpentine tolerance in the Mimulus guttatus Fischer ex DC. complex. S Afr J Sci 97:581–586
Jansa J, Smith FA, Smith FE (2008) Are there benefits of simultaneous colonization by different arbuscular mycorrhizal fungi? New Phytol 177:779–789
Ji BM, Bentivenga SP, Casper BB (2010) Evidence for ecological matching of whole AM fungal communities to the local plant-soil environment. Ecology 91:3037–3046
Johnson NC (2010) Resource stoichiometry elucidates the structure and function of arbuscular mycorrhizas across scales. New Phytol 185:631–647
Kishor PKB, Sangam S, Amrutha RN, Laxmi PS, Naidu KR, Rao KRSS, Rao S, Reddy KJ, Theriappan P, Sreenivasulu N (2005) Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: Its implications in plant growth and abiotic stress tolerance. Curr Sci 88:424–438
Kolář F, Štech M, Trávníček P, Rauchová J, Urfus T, Vít P, Kubešová M, Suda J (2009) Towards resolving the Knautia arvensis agg. (Dipsacaceae) puzzle: primary and secondary contact zones and ploidy segregation at landscape and microgeographic scales. Ann Bot-London 103:963–974
Koske RE, Gemma JN (1989) A modified procedure for staining roots to detect VA-mycorrhizas. Mycol Res 92:486–505
Krüger M, Stockinger H, Krüger C, Schüssler A (2009) DNA-based species level detection of Glomeromycota: one PCR primer set for all arbuscular mycorrhizal fungi. New Phytol 183:212–223
Lagrange A, Ducousso M, Jourand P, Majorel C, Amir H (2011) New insights into the mycorrhizal status of Cyperaceae from ultramafic soils in New Caledonia. Can J Microbiol 57:21–28
Lee BR, Muneer S, Avice JC, Jung WJ, Kim TH (2012) Mycorrhizal colonisation and P-supplement effects on N uptake and N assimilation in perennial ryegrass under well-watered and drought-stressed conditions. Mycorrhiza 22:525–534
Li T, Hu YJ, Hao ZP, Li H, Wang YS, Chen BD (2012) First cloning and characterization of two functional aquaporin genes from an arbuscular mycorrhizal fungus Glomus intraradices. New Phytol 197:617–630
Ludwig-Müller J (2010) Hormonal responses in host plants triggered by arbuscular mycorrhizal fungi. In: Koltai H, Kapulnik Y (eds) Arbuscular mycorrhizas: Physiology and function. Springer, pp. 169–190
Manoharan PT, Shanmugaiah V, Balasubramanian N, Gomathinayagam S, Sharma MP, Muthuchelian K (2010) Influence of AM fungi on the growth and physiological status of Erythrina variegata Linn. grown under different water stress conditions. Eur J Soil Biol 46:151–156
Marulanda A, Azcón R, Ruiz-Lozano JM (2003) Contribution of six arbuscular mycorrhizal fungal isolates to water uptake by Lactuca sativa plants under drought stress. Physiol Plantarum 119:526–533
Marulanda A, Porcel R, Barea JM, Azcón R (2007) Drought tolerance and antioxidant activities in lavender plants colonized by native drought-tolerant or drought-sensitive Glomus species. Microb Ecol 54:543–552
O’Dell R, Rajakaruna N (2011) Intraspecific variation, adaptation, and evolution. In: Harrison S, Rajakaruna N (eds) Serpentine: Evolution and ecology in a model system. UC Press, California, pp 97–137
Rajakaruna N, Bradfield GE, Bohm BA, Whitton J (2003) Adaptive differentiation in response to water stress by edaphic races of Lasthenia californica (Asteraceae). Int J Plant Sci 164:371–376
Römheld V, Kirkby EA (2010) Research on potassium in agriculture: needs and prospects. Plant Soil 335:155–180
Ruiz-Lozano JM, Azcón R, Gómez M (1995) Effects of arbuscular-mycorrhizal Glomus species on drought tolerance: Physiological and nutritional plant responses. Appl Environ Microb 61:456–460
Sambatti JBM, Rice KJ (2007) Functional ecology of ecotypic differentiation in the Californian serpentine sunflower (Helianthus exilis). New Phytol 175:107–119
Sauter A, Davies WJ, Hartung W (2001) The long-distance abscisic acid signal in the droughted plant: the fate of the hormone on its way from root to shoot. J Exp Bot 52:1991–1997
Schreiner RP, Bethlenfalvay GJ (1997) Plant and soil response to single and mixed species of arbuscular mycorrhizal fungi under fungicide stress. Appl Soil Ecol 7:93–102
Smith SE, Read DJ (2008) Mycorrhizal Symbiosis. Academic, Cambridge
Smith SE, Smith FA, Jakobsen I (2003) Mycorrhizal fungi can dominate phosphate supply to plants irrespective of growth responses. Plant Physiol 133:16–20
Smith SE, Facelli E, Pope S, Smith FA (2010) Plant performance in stressful environments: interpreting new and established knowledge of the roles of arbuscular mycorrhizas. Plant Soil 326:3–20
Štěpánek J (1997) Knautia L. – chrastavec. In: Slavík B (ed) Květena České republiky 6 [Flora of the Czech Republic 6]. Academia, Prague, pp 543–554
Subramanian KS, Charest C (1999) Acquisition of N by external hyphae of an arbuscular mycorrhizal fungus and its impact on physiological responses in maize under drought-stressed and well-watered conditions. Mycorrhiza 9:69–75
Subramanian KS, Santhanakrishnan P, Balasubramanian P (2006) Responses of field grown tomato plants to arbuscular mycorrhizal fungal colonization under varying intensities of drought stress. Sci Hortic 107:245–253
Trouvelot A, Kough JL, Gianinazzi-Pearson V (1986) Mesure du taux de mycorrhization VA d’un système radiculaire. Recherche de méthodes d’estimation ayant une signification fonctionnelle. In: Gianinazzi-Pearson V, Gianinazzi S (eds) Physiological and genetical aspects of mycorrhizae. INRA Paris, Dijon, pp 217–221
Wu QS, Xia RX, Zou YN, Wang GY (2007a) Osmotic solute responses of mycorrhizal citrus (Poncirus trifoliata) seedlings to drought stress. Acta Physiol Plant 29:543–549
Wu QS, Zou YN, Xia RX, Wang MY (2007b) Five Glomus species affect water relations of Citrus tangerine during drought stress. Bot Stud 48:147–154
Wu CA, Lowry DB, Nutter LI, Willis JH (2010) Natural variation for drought-response traits in the Mimulus guttatus species complex. Oecologia 162:23–33
Acknowledgments
The financial support of the Grant Agency of the Academy of Sciences of the Czech Republic (project KJB600050812) and the Grant Agency of Charles University in Prague (project 271011) is gratefully acknowledged. Additionally, this study was also supported by a long-term research development project no. RVO 67985939 (Academy of Sciences of the Czech Republic), by Charles University in Prague (project SVV 265203/2012), and by the Ministry of Agriculture of the Czech Republic (0002700604). The authors would also like to thank M. Albrechtová and her team at the Analytical laboratory of the Institute of Botany AS CR for their chemical analyses of plant biomass and soils, as well as T. Hájek (Institute of Botany AS CR, Třeboň) for the provision of and advice regarding the LI-COR, portable photosynthesis system. Thanks are also due to Prof. F. Asch and his team from the Institute for Plant Production and Agroecology in the Tropics and Subtropics at University of Hohenheim for providing the conjugate for ABA analysis. The advices of Z. Sýkorová and P. Kohout on AMF molecular determination, the support of members of the DNA laboratory of the Institute of Botany AS CR as well as the valuable comments of the editor and reviewers of the previous version of the manuscript are greatly appreciated.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Tatsuhiro Ezawa.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 480 kb)
Rights and permissions
About this article
Cite this article
Doubková, P., Vlasáková, E. & Sudová, R. Arbuscular mycorrhizal symbiosis alleviates drought stress imposed on Knautia arvensis plants in serpentine soil. Plant Soil 370, 149–161 (2013). https://doi.org/10.1007/s11104-013-1610-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-013-1610-7