Abstract
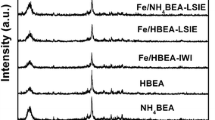
The catalytic decomposition of ammonia was studied over zeolite supported iron catalysts regarding the hot gas cleaning requirements of coal/biomass to liquid processes. Catalysts were prepared on different zeolite frameworks (HZY, HZβ and HZSM5 with SiO2 to Al2O3 ratios of 5.2, 38 and 280) and tested. Brighter grains in the SEM images of Fe/HZSM5 catalyst were noticed to be the iron aggregates on the external surface. Any indicative brighter grains were not detected in the SEM images of Fe/HZβ and Fe/HZY catalyst meaning that iron might be dispersed well and attached to the aluminum sites. The FeOx cluster size was considered to be dependent on the textural properties of the zeolites. The absence of α-iron (bcc) phase on the Fe/HZY catalyst upon reduction was interpreted as the strong interaction of iron with the aluminum sites of HZY. This interaction might provide the stabilization of FeO phase by preventing its further transformation to metallic iron. The reduction of stabilized FeO to metallic iron on Fe/HZY catalyst upon reaction seemed to involve a reaction between iron oxides and non-framework aluminum species like Al(OH)2+, Al(OH) +2 , AlO+, AlOOH etc. leading to iron aluminate (FeAl2O4)-like clusters. The highest activity over the Fe/HZβ catalyst might be due to the better hydrothermal stability of zeolite Hβ compared to zeolite HY regarding their aluminum contents. The role of the zeolite was probably to maintain good dispersion of active iron clusters without considering support effect on N–H bond breaking. Therefore, the best coordination environment for active iron species and improved iron cluster interactions can be ensured over zeolite Hβ framework.










Similar content being viewed by others
References
Kurkela E, Stahlberg P (1992) Fuel Process Technol 31:23–32
Wang W, Padban N, Ye Z, Andersson A, Bjerle I (1999) Ind Eng Chem Res 38:4175–4182
Rönkkönen H, Simell P, Reinikainen M, Krause O, Niemela MV (2010) Fuel 89:3272–3277
Hongrapipat J, Saw WL, Pang S (2012) Biomass Convers Biorefin 2(4):327–348
Xu CC, Donald J, Byambajav E, Ohtsuka Y (2010) Fuel 89:1784–1795
Corella J, Toledo JM, Padilla R (2005) Ind Eng Chem Res 44:2036–2045
Pinto F, Lopes H, Andre RN, Gulyurtlu I, Cabrita I (2007) Fuel 86:2052–2063
Björkman E (1991) Energy Fuels 5:753–760
Leppalahti J, Simell P, Kurkela E (1991) Fuel Process Technol 29:43–56
Simell PA, Hepola JO, Krasue AOI (1997) Fuel 76(12):1117–1127
Choudhary TV, Sivadinarayana C, Goodman DW (2001) Catal Lett 72(3–4):197–201
Yin SF, Xu BQ, Zhou XP, Au CT (2004) Appl Catal A 277:1–9
Dou B, Zhang M, Gao J, Shen W, Sha X (2002) Ind Eng Chem Res 41:4195–4200
Pansare SS, Torres W, Goodwin JG Jr (2007) Catal Commun 8:649–654
Leppalahti J (1993) Bioresour Technol 46:65–70
Mojtahedi W, Ylitalo M, Maunula T, Abbasian J (1995) Fuel Process Technol 45:221–236
Yasushi S, Yoshiharu M (2008) EP 1 872 852 A1
Torres W, Pansare SS, Goodwin JG Jr (2007) Catal Rev 49(4):407–456
Zheng W, Zhang J, Xu H (2007) Catal Lett 119:311–318
Ohtsuka Y, Xu C, Kong D, Tsubouchi N (2004) Fuel 83:685–692
Donald J, Xu CC, Hashimoto H, Byambajav E, Ohtsuka Y (2010) Appl Catal A 375:124–133
Simell P, Kurkela E, Stahlberg P, Hepola J (1996) Catal Today 27(1–2):55–62
Tsubouchi N, Hashimoto H, Ohtsuka Y (2005) Catal Lett 105(3–4):203–208
Xu C, Tsubouchi N, Hashimoto H, Ohtsuka Y (2005) Fuel 84:1957–1967
Liu H (2013) Ammonia synthesis catalysts: innovation and practice, Chapter 1, World Scientific, p 16. http://www.worldscientific.com/doi/pdf/10.1142/9789814355780_fmatter
Li XK, Ji WJ, Zhao J, Wang SJ, Au CT (2005) J Catal 236:181–189
Liu H, Wang H, Shen J, Sun İ, Liu H (2008) Catal Today 131:444–449
Hashimoto K, Toukai N (2000) J Mol Catal A 161:171–178
Chen J, Zhu ZH, Wang S, Ma Q, Rudolph V, Lu GQ (2010) Chem Eng J 156:404–410
Baranak M, Gürünlü B, Sarıoğlan A, Ataç Ö, Atakül H (2013) Catal Today 207:57–64
Storck S, Bretinger H, Maier WF (1998) Appl Catal A 174:137–146
Huang Z, Su JF, Guo YH, Su XQ, Teng LJ (2009) Chem Eng Commun 196:969–986
Raul FL (2005) In: Auerbach SM, Carrado KA, Dutta PK (eds) Handbook of zeolite science and technology, 1st edn. Marcel Dekker, Inc., NewYork
Van Ness DJS (2010) Ph.D. thesis, UMI Number: 3433620:3. (http://gradworks.umi.com/34/33/3433620.html)
Eliana Misi SE, Ramli A, Rahman FH (2011) J Appl Sci 11(8):1297–1302
Kazansky VB (1988) Catal Today 3:367–372
Ren-Yuan T, Su Z, Chengyu W, Dongbai L, Liwu L (1987) J Catal 106:440–448
Strongin DR, Somorjai GA (1991) In: Jennings JR (ed) Catalytic ammonia synthesis: fundementals and practice, 1991st edn. Springer, New York
Hansgen DA, Vlachos DG, Chen JG (2010) Nat Chem 2:484–489
Lee HT, Rhee HK (1999) Catal Lett 61:71–76
Yuen S, Chen Y, Kubsh JE, Dumesic JA, Topsoe N, Topsoe H (1982) J Phys Chem 86:3022–3032
Lobree LJ, Hwang IC, Reimer JA, Bell AT (1999) J Catal 186:242–253
Dong H, Xie M, Xu J, Li M, Peng L, Guo X, Ding W (2011) Chem Commun 47(13):4019–4021
Park JY, Lee YJ, Khanna PK, Jun KW, Bae JW, Kim YH (2010) J Mol Catal A 323:84–90
Greenwood NN, Earnshaw A (1997) Chemistry of the elements, 2nd edn. Butterworth-Heinemann, Oxford. ISBN 0080379419
Li G, Pidko EA, Santen RAV, Li C, Hensen EJM (2012) J Phys Chem 117:413–426
Guo W, Vlachos DG (2015) Patched bimetallic surfaces are active catalysts for ammonia decomposition. Nat Commun. doi:10.1038/ncomms9619
Fickel DW, D’Addio E, Lauterbach JA, Lobo RF (2011) Appl Catal B 102:441–448
Acknowledgments
The authors greatly acknowledge to “The Scientific and Technological Research Council of Turkey (TÜBİTAK)” for supporting of “Liquid Fuel Production from Biomass and Coal Blends” project (Contract Code: 108G043) in which this study was carried out. The authors would also like to thank the State Planning Organization of Turkey for their laboratory infrastructure funding (Project: Excellency Center for Gas Technologies, 5112101).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Durak-Çetin, Y., Sarıoğlan, Ş., Sarıoğlan, A. et al. The effect of support type on the activity of zeolite supported iron catalysts for the decomposition of ammonia. Reac Kinet Mech Cat 118, 683–699 (2016). https://doi.org/10.1007/s11144-016-0981-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-016-0981-1