Abstract
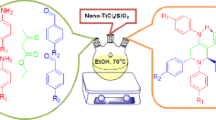
1-Amidoalkyl-2-naphthols, 1-carbamatoalkyl-2-naphthols, and 1-(α-aminoalkyl)-2-naphthols have been prepared by three-component reaction of 2-naphthol, aromatic aldehydes, and NH compounds, i.e. amides, carbamates, and secondary amines, respectively, in the presence of a catalytic amount of nanocrystalline TiO2–HClO4. In addition, 12-aryl-8,9,10,12-tetrahydrobenzo[a]-xanthen-11-one derivatives have been synthesized by reaction of 2-naphthol, aromatic aldehydes, and dimedone in the presence of the same nano catalyst. These reactions were studied under solvent-free conditions. This white acidic heterogeneous catalyst is very stable under the reaction conditions and was reused several times without significant loss of activity.








Similar content being viewed by others
References
M. Crippa, E. Callone, M. D’Arienzoa, K. Müllerb, S. Polizzi, L. Wahba, F. Morazzoni, R. Scotti, Appl. Catal. B 104, 282–290 (2011)
B.-H. Leea, T. Nakayama, Y. Tokoi, T. Suzuki, K. Niihara, J. Alloy. Compd. 509, 1231–1235 (2011)
Z. Zhao, J. Fan, M. Xie, Z. Wang, J. Clean. Prod. 17, 1025–1029 (2009)
F. Shirini, S.V. Atghia, M.G. Jirdehi, Catal Commun 18, 5–10 (2012)
J. Zhu, H. Bienayme, Multicomponent reactions (Wiley–VCH, Weinheim, 2005)
A. Dömling, Chem. Rev. 106, 17–89 (2006)
S. Jimenez-Alonso, H. Chavez, A. Estevez-Braan, A. Ravelo, G. Feresin, A. Tapia, Tetrahedron 64, 8938–8942 (2008)
D. Seebach, J.L. Matthews, J. Chem. Soc. 21, 2015–2022 (1997)
Y.F. Wang, T. Izawa, S. Kobayashi, M. Ohno, J. Am. Chem. Soc. 104, 6465–6466 (1982)
S. Knapp, Chem. Rev. 95, 1859–1876 (1995)
E. Juaristi, Enantioselective synthesis of β-amino acids (Wiley, New York, 1997)
J.P. Poupelin, G. Saint-Ruf, O. Foussard-Blanpin, G. Narcisse, G. Uchida-Ernouf, R. Lacroix, Eur. J. Med. Chem. 13, 67–71 (1978)
R.W. Lambert, J.A. Martin, J.H. Merrett, K.E.B. Parkes, G.J. Thomas, PCT Int. Appl. WO 9706178, 1997, 1-80
T. Hideo, J. Teruomi, Jpn. Patent 56005480, 1981
H.R. Shaterian, M. Mohammadnia, F. Moradi, J. Mol. Liq. 172, 88–92 (2012)
H.R. Shaterian, M. Mohammadnia, J. Mol. Liq. 173, 55–61 (2012)
N.P. Selvam, P.T. Perumal, Tetrahedron Lett. 47, 7481–7483 (2006)
S.B. Patil, P.R. Singh, M.P. Surpur, S.D. Samant, Ultrason. Sonochem. 14, 515–518 (2007)
H.R. Shaterian, H. Yarahmadi, M. Ghashang, Bioorg. Med. Chem. Lett. 18, 788–792 (2008)
S.B. Patil, P.R. Singh, M.P. Surpur, S.D. Samant, Synth. Commun. 37, 1659–1664 (2007)
A. Dorehgiraee, H. Khabazzade, K. Saidi, ARKIVOC 7, 303–310 (2009)
A.R. Hajipour, Y. Ghayeb, N. Sheikhan, A.E. Ruoho, Tetrahedron Lett. 50, 5649–5651 (2009)
M. Wang, Y. Liang, T.T. Zhang, J.J. Gao, Chin. Chem. Lett. 23, 65–68 (2012)
A. Zali, A. Shokrolahi, Chin. Chem. Lett. 23, 269–272 (2012)
A. Zare, A. Hasaninejad, A. Salimi Beni, A.R. Moosavi-Zare, M. Merajoddin, E. Kamali, M. Akbari-Seddigh, Z. Parsaee, Sci. Iran. C 18, 433–438 (2011)
H.R. Shaterian, A. Hosseinian, M. Ghashang, Tetrahedron Lett. 49, 5804–5806 (2008)
D. Kundu, A. Majee, A. Hajra, Catal. Commun. 11, 1157–1159 (2010)
A. Kumar, M.K. Gupta, M. Kumar, Tetrahedron Lett. 51, 1582–1584 (2010)
M.R. Saidi, N. Azizi, M.R. Naimi-Jamal, Tetrahedron Lett. 42, 8111–8113 (2001)
S.D. Dindulkar, V.G. Puranik, Y.T. Jeong, Tetrahedron Lett. 53, 4376–4380 (2012)
A. Ravindran, R. Srivastava, Chin. J. Catal. 32, 1597–1603 (2011)
G.C. Nandi, S. Samai, R. Kumar, M.S. Singh, Tetrahedron 65, 7129–7134 (2009)
A. Khazaei, M.A. Zolfigol, A.R. Moosavi-Zare, A. Zare, M. Khojasteh, Z. Asgari, V. Khakyzadeh, A. Khalafi-Nezhad, Catal. Commun. 20, 54–57 (2012)
H.-J. Wang, X.-Q. Ren, Y–.Y. Zhang, Z.-H. Zhang, J. Braz. Chem. Soc. 20, 1939–1943 (2009)
J.M. Khurana, D. Magoo, Tetrahedron Lett. 50, 4777–4780 (2009)
Acknowledgments
We are grateful to the University of Sistan and Baluchestan Research Council for partial support of this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shaterian, H.R., Mohammadnia, M. Nanocrystalline TiO2–HClO4 catalyzed three-component preparation of derivatives of 1-amidoalkyl-2-naphthol, 1-carbamato-alkyl-2-naphthol, 1-(α-aminoalkyl)-2-naphthol, and 12-aryl-8,9,10,12-tetrahydrobenzo[a]-xanthen-11-one. Res Chem Intermed 39, 4221–4237 (2013). https://doi.org/10.1007/s11164-012-0938-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-012-0938-6