Abstract
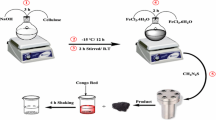
In this study, zinc oxide was immobilized on magnetite nanoparticles by chemical method and it was used as an adsorbent to remove reactive black 5 (RB5) dye from aqueous solution. The removal efficiency of RB5 was studied as the function of adsorbent dosage, pH, initial RB5 concentration, H2O2, and ionic strength (sodium carbonate, sodium bicarbonate, sodium sulfate, and sodium chloride). Removal efficiency of RB5 by ZnO–Fe3O4 was greater than that by ZnO and Fe3O4 in similar conditions. Maximum adsorption of ZnO–Fe3O4 was obtained at neutral pH, and adsorption capacity was estimated to be 22.1 mg/g. Adsorption kinetic study revealed that the pseudo-second-order model better described the removal rate than the pseudo-first-order model. Adsorption isotherm was analyzed by both Langmuir and Freundlich equations, and results showed that it was better described by the Langmuir equation. The removal efficiency of RB5 was increased with increasing initial H2O2 concentrations from 2 to 5 mM but was decreased above 5 mM. The adsorption capacities of RB5 was increased in the presence of NaCl but was greatly decreased in the presence of bicarbonate, carbonate, and sulfate ion. Adsorption activity of RB5 by ZnO–Fe3O4 composite was maintained even after five successive cycles, suggesting a promising adsorbent for wastewater-contaminated organic dyes.











Similar content being viewed by others
References
Ai, L., Zhang, C., & Chen, Z. (2011). Removal of methylene blue from aqueous solution by a solvothermal-synthesized graphene/magnetite composite. Journal of Hazardous Materials, 192, 1515–1524.
Al-Degs, Y., Khraisheh, M. A. M., Allen, S. J., & Ahmad, M. N. (2000). Effect of carbon surface chemistry on the removal of reactive dyes from textile effluent. Water Research, 34, 927–935.
Asgher, M. (2012). Biosorption of reactive dyes: a review. Water, Air, & Soil Pollution, 223, 2417–2435.
Azizian, S. (2004). Kinetic models of sorption: a theoretical analysis. Journal of Colloid and Interface Science, 276, 47–52.
Barron-Zambrano, J., Szygula, A., Ruiz, M., Sastre, A. M., & Guibal, E. (2010). Biosorption of reactive black 5 from aqueous solutions by chitosan: column studies. Journal of Environmental Management, 91, 2669–2675.
Behnajady, M. A., Modirshahla, N., Hamzavi, R., Behnajady, M. A., Modirshahla, N., & Hamzavi, R. (2006). Kinetic study on photocatalytic degradation of C.I. acid yellow 23 by ZnO photocatalyst. Journal of Hazardous Materials, 133, 226–232.
Camp, S. R., & Sturrock, P. E. (1990). The identification of the derivatives of C.I. reactive blue 19 in textile wastewater. Water Research, 24, 1275–1278.
Chatterjee, S., Chatterjee, S., Chatterjee, B. P., & Guha, A. K. (2007). Adsorptive removal of congo red, a carcinogenic textile dye by chitosan hydrobeads: binding mechanism, equilibrium and kinetics. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 299, 146–152.
Chen, C., Gunawan, P., & Xu, R. (2011). Self-assembled Fe3O4-layered double hydroxide colloidal nanohybrids with excellent performance for treatment of organic dyes in water. Journal of Materials Chemistry, 21, 1218–1225.
Choi, H.-D., Shin, M.-C., Kim, D.-H., Jeon, C.-S., & Baek, K. (2008). Removal characteristics of reactive black 5 using surfactant-modified activated carbon. Desalination, 223, 290–298.
Daneshvar, N., Khataee, A. R., Rasoulifard, M. H., & Pourhassan, M. (2007a). Biodegradation of dye solution containing malachite green: optimization of effective parameters using Taguchi method. Journal of Hazardous Materials, 143, 214–219.
Daneshvar, N., Rasoulifard, M. H., Khataee, A. R., & Hosseinzadeh, F. (2007b). Removal of C.I. acid orange 7 from aqueous solution by UV irradiation in the presence of ZnO nanopowder. Journal of Hazardous Materials, 143, 95–101.
Elizalde-Gonzalez, M. P., & Hernandez-Montoya, V. (2009). Removal of acid orange 7 by guava seed carbon: a four parameter optimization study. Journal of Hazardous Materials, 168, 515–522.
Elwakeel, K. Z. (2009). Removal of reactive black 5 from aqueous solutions using magnetic chitosan resins. Journal of Hazardous Materials, 167, 383–392.
Eren, Z., & Acar, F. N. (2006). Adsorption of reactive black 5 from an aqueous solution: equilibrium and kinetic studies. Desalination, 194, 1–10.
Fagundes-Klen, M., Cervelin, P., Veit, M., Cunha Gonçalves, G., Bergamasco, R., & Silva, F. (2012). Adsorption kinetics of blue 5G dye from aqueous solution on dead floating aquatic macrophyte: effect of pH, temperature, and pretreatment. Water, Air, & Soil Pollution, 223, 4369–4381.
Ferrero, F. (2007). Dye removal by low cost adsorbents: hazelnut shells in comparison with wood sawdust. Journal of Hazardous Materials, 142, 144–152.
Gaikwad, R. W., & Kinldy, S. A. M. (2009). Studies on auramine dye adsorption on psidium guava leaves. Korean Journal of Chemical Engineering, 26, 102–107.
Geng, Z., Lin, Y., Yu, X., Shen, Q., Ma, L., Li, Z., et al. (2012). Highly efficient dye adsorption and removal: a functional hybrid of reduced graphene oxide-Fe3O4 nanoparticles as an easily regenerative adsorbent. Journal of Materials Chemistry, 22, 3527–3535.
Gulnaz, O., Kaya, A., & Dincer, S. (2006). The reuse of dried activated sludge for adsorption of reactive dye. Journal of Hazardous Materials, 134, 190–196.
Gupta, V. K., Ali, I., Suhas, & Mohan, D. (2003). Equilibrium uptake and sorption dynamics for the removal of a basic dye (basic red) using low-cost adsorbents. Journal of Colloid and Interface Science, 265, 257–264.
Horwitz, W. (2000). Standard methods for the examination of water and wastewater (20th ed.). Washington, D.C.: APHA.
Ip, A. W. M., Barford, J. P., & Mckay, G. (2010). A comparative study on the kinetics and mechanisms of removal of reactive black 5 by adsorption onto activated carbons and bone char. Chemical Engineering Journal, 157, 434–442.
Khataee, A. R., & Kasiri, M. B. (2010). Photocatalytic degradation of organic dyes in the presence of nanostructured titanium dioxide: influence of the chemical structure of dyes. Journal of Molecular Catalysis A: Chemical, 328, 8–26.
Khataee, A. R., Fathinia, M., Aber, S., & Zarei, M. (2010). Optimization of photocatalytic treatment of dye solution on supported TiO2 nanoparticles by central composite design: intermediates identification. Journal of Hazardous Materials, 181, 886–897.
Nataraj, S. K., Hosamani, K. M., & Aminabhavi, T. M. (2009). Nanofiltration and reverse osmosis thin film composite membrane module for the removal of dye and salts from the simulated mixtures. Desalination, 249, 12–17.
Nigam, P., Armour, G., Banat, I. M., Singh, D., & Marchant, R. (2000). Physical removal of textile dyes from effluents and solid-state fermentation of dye-adsorbed agricultural residues. Bioresource Technology, 72, 219–226.
Patterson, A. (1939). The Scherrer formula for X-ray particle size determination. Physical Review, 56, 978.
Qiu-Yue, Z., Ping, Y., Yan, W., & Lei, J. (2010). Study on adsorption of Congo red in water on calcined oyster shell powder. Technology & Development of Chemical Industry, 48–50.
Raghu, S., & Ahmed Basha, C. (2007). Chemical or electrochemical techniques, followed by ion exchange, for recycle of textile dye wastewater. Journal of Hazardous Materials, 149, 324–330.
Regan, B. O., & Schwartz, D. T. (1995). Efficient photo-hole injection from adsorbed cyanine dyes into electrodeposited copper(I) thiocyanate thin films. Chemistry of Materials, 7, 1349–1354.
Rongcheng, W., & Jiuhui, Q. (2004). Removal of azo dye from water by magnetite adsorption-fenton oxidation. Water Environment Research, 76, 2637–2642.
Samarghandi, M. R., Azizian, S., Shirzad-Siboni, M., Jafari, S. J., & Rahimi, S. (2011). Removal of divalent nickel from aqueous solutions by adsorption onto modified holly sawdust: equilibrium and kinetics. Journal of Environmental Health Science and Engineering, 8, 181–188.
Sen, T., Afroze, S., & Ang, H. M. (2011). Equilibrium, kinetics and mechanism of removal of methylene blue from aqueous solution by adsorption onto pine cone biomass of pinus radiata. Water, Air, & Soil Pollution, 218, 499–515.
Sengila, I. A., & Ozacarb, M. (2009). The decolorization of C.I. reactive black 5 in aqueous solution by electrocoagulation using sacrificial iron electrodes. Journal of Hazardous Materials, 161, 1369–1376.
Shirzad-Siboni, M., Samarghandi, M., Yang, J.-K., & Lee, S.-M. (2011a). Photocatalytic removal of reactive black-5 dye from aqueous solution by UV irradiation in aqueous TiO2: equilibrium and kinetics study. Journal of Advanced Oxidation Technologies, 14, 302–307.
Shirzad-Siboni, M., Samarghandi, M. R., Azizian, S., Kim, W. G., & Lee, S. M. (2011b). The removal of hexavalent chromium from aqueous solutions using modified holly sawdust: equilibrium and kinetics studies. Environmental Engineering Research, 16, 1–6.
Shirzad-Siboni, M., Farrokhi, M., Darvishi Cheshmeh Soltani, R., Khataee, A. & Tajassosi, S. (2013a). Photocatalytic reduction of hexavalent chromium over ZnO nanorods immobilized on kaolin. Industrial & Engineering Chemistry Research.
Shirzad-Siboni, M., Jafari, S.-J., Farrokhi, M., & Yang, J. K. (2013b). Removal of phenol from aqueous solutions by activated red mud: equilibrium and kinetics studies. Environmental Engineering Research, 18, 247–252.
Shirzad-Siboni, M., Jafari, S. J., Giahi, O., Kim, I., Lee, S.-M., & Yang, J.-K. (2014a). Removal of acid blue 113 and reactive black 5 dye from aqueous solutions by activated red mud. Journal of Industrial and Engineering Chemistry, 20, 1432–1437.
Shirzad-Siboni, M., Khataee, A., & Joo, S. W. (2014b). Kinetics and equilibrium studies of removal of an azo dye from aqueous solution by adsorption onto scallop. Journal of Industrial and Engineering Chemistry, 20, 610–615.
Shirzad-Siboni, M., Khataee, A., Vafaei, F. & Joo, S. (2014b). Comparative removal of two textile dyes from aqueous solution by adsorption onto marine-source waste shell: kinetic and isotherm studies. Korean Journal of Chemical Engineering, 1–9.
Singh, S., Barick, K., & Bahadur, D. (2013). Fe3O4 embedded ZnO nanocomposites for the removal of toxic metal ions, organic dyes and bacterial pathogens. Journal of Materials Chemistry A, 1, 3325–3333.
Sun, D., Zhang, X., Wu, Y., & Liu, X. (2010). Adsorption of anionic dyes from aqueous solution on fly ash. Journal of Hazardous Materials, 181, 335–342.
Sun, H., Cao, L., & Lu, L. (2011). Magnetite/reduced graphene oxide nanocomposites: one step solvothermal synthesis and use as a novel platform for removal of dye pollutants. Nano Research, 4, 550–562.
Uzun, İ. (2006). Kinetics of the adsorption of reactive dyes by chitosan. Dyes and Pigments, 70, 76–83.
Walker, G. M., Hansen, L., Hanna, J. A., & Allen, S. J. (2003). Kinetics of a reactive dye adsorption onto dolomitic sorbents. Water Research, 37, 2081–2089.
Wang, S., Boyjoo, Y., Choueib, A., & Zhu, Z. H. (2005). Removal of dyes from aqueous solution using fly ash and red mud. Water Research, 39, 129–138.
Wang, N., Zhu, L., Wang, M., Wang, D., & Tang, H. (2010). Sono-enhanced degradation of dye pollutants with the use of H2O2 activated by Fe3O4 magnetic nanoparticles as peroxidase mimetic. Ultrasonics Sonochemistry, 17, 78–83.
Wang, L., Li, J., Jiang, Q., & Zhao, L. (2012). Water-soluble Fe3O4 nanoparticles with high solubility for removal of heavy-metal ions from waste water. Dalton Transactions, 41, 4544–4551.
Wu, Eiteman, M., & Law. (1998). Evaluation of membrane filtration and ozonation processes for treatment of reactive-dye wastewater. Journal of Environmental Engineering, 124, 272–277.
Wu, Q., Feng, C., Wang, C., & Wang, Z. (2013). A facile one-pot solvothermal method to produce superparamagnetic graphene–Fe3O4 nanocomposite and its application in the removal of dye from aqueous solution. Colloids and Surfaces B: Biointerfaces, 101, 210–214.
Xie, G., Xi, P., Liu, H., Chen, F., Huang, L., Shi, Y., et al. (2012). A facile chemical method to produce superparamagnetic graphene oxide-Fe3O4 hybrid composite and its application in the removal of dyes from aqueous solution. Journal of Materials Chemistry, 22, 1033–1039.
Xue, Y., Hou, H., & Zhu, S. (2009). Adsorption removal of reactive dyes from aqueous solution by modified basic oxygen furnace slag: isotherm and kinetic study. Chemical Engineering Journal, 147, 272–279.
Yagub, M., Sen, T., & Ang, H. M. (2012). Equilibrium, kinetics, and thermodynamics of methylene blue adsorption by pine tree leaves. Water, Air, & Soil Pollution, 223, 5267–5282.
Zhu, M.-X., Lee, L., Wang, H.-H., & Wang, Z. (2007). Removal of an anionic dye by adsorption/precipitation processes using alkaline white mud. Journal of Hazardous Materials, 149, 735–741.
Acknowledgments
The authors thank the Guilan, Alborz, and Iran Universities of Medical Sciences of Iran for their contributions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Farrokhi, M., Hosseini, SC., Yang, JK. et al. Application of ZnO–Fe3O4 Nanocomposite on the Removal of Azo Dye from Aqueous Solutions: Kinetics and Equilibrium Studies. Water Air Soil Pollut 225, 2113 (2014). https://doi.org/10.1007/s11270-014-2113-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-014-2113-8