Abstract
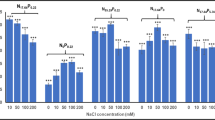
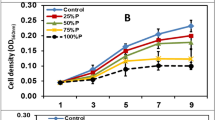
Two microalgae strains including Chlorella vulgaris and Acutodesmus obliquus were grown on BG11 medium with salinity stress ranging from 0.06 to 0.4 M NaCl. Highest lipid content in C. vulgaris and A. obliquus was 49 and 43% in BG11 amended with 0.4 M NaCl. The microalgal strains C. vulgaris and A. obliquus grow better at 0.06 M NaCl concentration than control condition. At 0.06 M NaCl, improved dry biomass content in C. vulgaris and A. obliquus was 0.92 and 0.68 gL−1, respectively. Stress biomarkers like reactive oxygen species, antioxidant enzyme catalase, and ascorbate peroxidase were also lowest at 0.06 M NaCl concentration revealing that both the microalgal strains are well acclimatized at 0.06 M NaCl concentration. The fatty acid composition of the investigated microalgal strains was also improved by increased NaCl concentration. At 0.4 M NaCl, palmitic acid (37%), oleic acid (15.5%), and linoleic acid (20%) were the dominant fatty acids in C. vulgaris while palmitic acid (54%) and stearic acid (26.6%) were major fatty acids found in A. obliquus. Fatty acid profiling of C. vulgaris and A. obliquus significantly varied with salinity concentration. Therefore, the study showed that salt stress is an effective stress that could increase not only the lipid content but also improved the fatty acid composition which could make C. vulgaris and A. obliquus potential strains for biodiesel production.





Similar content being viewed by others
References
An M, Mou S, Zhang X, Zheng Z, Ye N, Wang D, Zhang W, Miao J (2013) Expression of fatty acid desaturase genes and fatty acid accumulation in Chlamydomonas sp. ICE-L under salt stress. Bioresour Technol 149:77–83. doi:10.1016/j.biortech.2013.09.027
Alyabyev AJ, Loseva NL, Gordon LK, Andreyeva IN, Rachimova GG, Tribunskih VI, Ponomareva AA, Kemp RB (2007) The effect of changes in salinity on the energy yielding processes of Chlorella vulgaris and Dunaliella maritima cells. Thermochim Acta 458:65–70 doi.org/10.1016/j.tca.2007.03.003
Arias-Penarands MT, Cristiani-Urbina E, Montes-Horcasitas CM, Esparza-Garcia F, Torzillo G, Canizares-Villanueva RO (2013) Scenedesmus incrassatulus CLHE-Si01: a potential source of renewable lipid for high quality biodiesel production. Bioresour Technol 140:158–164. doi:10.1016/j.biortech.2013.04.080
Azachi M, Sadka A, Fisher M, Goldshlag P, Gokhman I, Zamir A (2002) Salt induction of fatty acid elongase and membrane lipid modifications in the extreme halotolerant alga Dunaliella salina. Plant Physiol 129:1320–1329. doi:10.1104/pp.001909
Bajwa K, Bishnoi NR (2015) Osmotic stress induced by salinity for lipid overproduction in batch culture of Chlorella pyrenoidosa and effect on others physiological as well as physicochemical attributes. J. Algal Biomass Utln. 6:26–34
Bartley ML, Boeing WJ, Dungan BN, Holguin FO, Schaub T (2014) pH effects on growth and lipid accumulation of the biofuel microalgae Nannochloropsis salina and invading organisms. 1431–1437. doi:10.1007/s10811-013-0177-2
Battah MG, Ayoty YM, Esmael AE, Abd El-Ghany SE (2014) Effect of different concentrations of sodium nitrate, sodium chloride, and ferrous sulphate on the growth and lipid content of Chlorella vulgaris. Journal of Agricultural Technology 10(2):339–353
Bhattacharya S, Maurya R, Mishra SK, Ghosh T, Patidar SK, Paliwal C, Mishra S (2016) Solar driven mass cultivation and the extraction of lipids from Chlorella variabilis: a case study. Algal Res 14:137–142
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Phys 37(8):911–917. doi:10.1139/o59-099
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254. doi:10.1016/0003-2697(76)90527-3
Campenni L, Nobre BP, Santos CA, Oliveira A, Aires BM, Palavra A, Gouveia L (2013) Carotenoid and lipid production by the autotrophic microalga Chlorella protothecoides under nutritional, salinity, and luminosity stress conditions. Appl Microbiol Biotechnol 97(3):1383–1393. doi:10.1007/s00253-012-4570-6
Chi X, Yang Q, Zhao F, Qin S, Yang Y, Shen J, Lin H (2008) Comparative analysis of fatty acid desaturases in cyanobacterial genomes. Comp Funct Genomics:1–25. doi:10.1155/2008/284508
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25:294–306. doi:10.1016/j.biotechadv.2007.02.001
Chittra Y, Benjamas C (2011) Effect of nitrogen, salt, and iron content in the growth medium and light intensity on lipid production by microalgae isolated from freshwater sources in Thailand. Bioresour Technol 102:3034–3040. doi:10.1016/j.biortech.2010.10.013
Cho S, Dukhaeng L, Thao TL, Parrk S, Kwan YO, Lee T (2011) Effects of carbon and nitrogen sources on fatty acid contents and composition in the green microalga, Chlorella sp. 227. J Microbiol Biotechnol 21(10):1073–1080. doi:10.4014/jmb.1103.03038
Chokshi K, Pancha I, Trivedi K, George B, Maurya R, Ghosh A, Mishra S (2015) Biofuel potential of the newly isolated microalgae Acutodesmus dimorphus under temperature induced oxidative stress conditions. Bioresour Technol 180:161–171. doi:10.1016/j.biortech.2014.12.102
Chun YC, Xin QZ, Hong WY, Shih HH, Chieh LC, Duu JL, Feng WB, Jo SC (2013) Microalgae-based carbohydrates for biofuel production. Biochem Eng J 78:1–10. doi:10.1016/j.bej.2013.03.006
Chun YC, Kuei LY, Rifka A, Duu-JL J-SC (2011) Cultivation, photobioreactor design and harvesting of microalgae for biodiesel production: a critical review. Bioresour Technol 102:71–81. doi:10.1016/j.biortech.2010.06.159
Curtis A, Bruce B (2013) Enhancing lipid production of the marine diatom Chaetoceros gracilis: synergistic interactions of sodium chloride and silicon. J Appl Phycol. doi:10.1007/s10811-013-0156-7
Dahmen MBI, Chtourou H, Rezgui F, Sayadi S, Dhouib A (2016) Salinity stress increases lipid, secondary metabolites and enzyme activity in Amphora subtropica and Dunaliella sp. for biodiesel production. Bioresource Technology 816–825. doi:10.1016/j.biortech.2016.07.022
Dhup S, Dhawan V (2014) Effect of nitrogen concentration on lipid productivity and fatty acid composition of Monoraphidium sp. Bioresour Technol 152:572–575. doi:10.1016/j.biortech.2013.11.068
Dittami SM, Gravot A, Renault D, Goulitquer S, Eggert A, Bouchereau A, Boyen C, Tonon T (2011) Integrative analysis of metabolite and transcript abundance during the short-term response to saline and oxidative stress in the brown alga Ectocarpus Siliculosus. Plant Cell Environ 34:629–642. doi:10.1111/j.1365-3040.2010.02268.x
El-Sheekh M, Abomohra AEF, Hanelt D (2012) Optimization of biomass and fatty acid productivity of Scenedesmus obliquus as a promising microalga for biodiesel production. World J Microbiol Biotechnol. doi:10.1007/s11274-012-1248-2
Eyster HC, Brown TE, Tanner HA (1958) Mineral requirements for Chlorella Pyrenoidosa under autotrophic and heterotrophic conditions. In: Lamb CA, Bentley OJ, Beattie JM (eds) Trace elements. Academic Press, New York, pp 157–191
Fan J, Cui Y, Wan M, Wang W, Li Y (2014) Lipid accumulation and biosynthesis genes response of the oleaginous Chlorella pyrenoidosa under three nutrition stressors. Biotechnol Biofuels 7:17
Fen L, Chung P, Mei C (2013) The effects of nitrogen sources and temperature on cell growth and lipid accumulation of microalgae. International Biodeterioration & Biodegradation 85:506–510. doi:10.1016/j.ibiod.2013.05.016
Francisco ÉC, Neves DB, Jacob-Lopes E, Franco TT (2010) Microalgae as feedstock for biodiesel production: carbon dioxide sequestration, lipid production and biofuel quality. J Chem Technol Biotechnol 85:395–403. doi:10.1002/jctb.2338
Freddy G, Virginie M, Lionel U, Gerard T (2009) Combined effects of irradiance level and carbon source on fatty acid and lipid class composition in the microalga Pavlova lutheri commonly used in mariculture. J Exp Mar Biol Ecol 369:136–143. doi:10.1016/j.jembe2008.11.009
Gill PK, Sharma AD, Singh P, Bhullar SS (2002) Osmotic stress induced changes in germination, growth and soluble sugar contents of Sorghum bicolor (L.) Moench seeds under various abiotic stresses. Plant Physiol 128:12–25
Gonzalez LCV (2010) Protein measurements of microalgal and cyanobacterial biomass. Bioresour Technol 101:7587–7591. doi:10.1016/j.biortech.2010.04.077
Griffiths MJ, Harrison STL (2009) Lipid productivity as a key characteristic for choosing algal species for biodiesel production. J Appl Phycol. 493–507. doi:10.1007/s10811-008-9392-7
Hallenbeck PC, Benemann JR (2002) Biological hydrogen production: fundamentals and limiting processes. Int J Hydrogen Energ 27:1185–1193. doi:10.1016/S0360-3199(02)00131-3
Hiremath S, Mathad P (2010) Impact of salinity on the physiological and biochemical traits of Chlorella vulgaris Beijerinck. J. Algal Biomass Utln. 1(2):51–59
Hong WY, Chen H, Chun YC, Shih HH, J Lee D, Jo-Shu C (2013) Microalgae-based biorefinery –from biofuels to natural products. Bioresour Technol 135:166–174. doi:10.1016/j.biortech.2012.10.099
Hu Q, Sommerfeld M, Jarvis E, Ghirardi M, Posewitz M, Seibert M, Darzins A (2008) Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J 54(4):621–639. doi:10.1111/j.1365-313x.2008.03492.x
Jayanta T, Chandra MK, Chandra BG (2012) Growth, total lipid content and fatty acid profile of a native strain of the freshwater oleaginous microalgae ankistrodesmus falcatus (Ralf) grown under salt stress condition. International Research Journal of Biological Sciences 1(8):27–35
Jiang H, Gao K (2004) Effects of lowering temperature during culture on the production of polyunsaturated fatty acids in the marine diatom Phaeodactylum tricornutum (Bacillariophyceae). J Phycol 40:651–654
Jin L, Junchao H, Zheng S, Yujuan Z, Yue J, Feng C (2011) Differential lipid and fatty acid profiles of photoautotrophic and heterotrophic Chlorella zofingiensis: assessment of algal oils for biodiesel production. Bioresour Technol 102:106–110. doi:10.1016/j.biortech.2010.06.017
Kan G, Shi C, Wang X, Xie Q, Wang M, Wang X, Miao J (2012) Acclimatory responses to high-salt stress in Chlamydomonas (Chlorophyta, Chlorophyceae) from Antarctica. Acta Oceanol Sin 31:116–124. doi:10.1007/s13131-012-0183-2
Karpagam R, Preeti R, Raj KJ, Saranya S, Ashokkumar B, Varalakshmi P (2015) Fatty acid biosynthesis from a new isolate Meyerella sp. N4: molecular characterization, nutrient starvation, and fatty acid profiling for lipid enhancement. Energy Fuel 29:143–149. doi:10.1021/ef501969a
Kato M, Shimizu S (1987) Chlorophyll metabolism in higher plants. VII. Chlorophyll degradation in senescing tobacco leaves; phenolic-dependent peroxidative degradation. Can J Bot 65:729–735. doi:10.1139/b87-097
Kirk JTO, Allen RL (1965) Dependence of chloroplast pigment synthesis on protein synthesis effects of actilione. Biochem Biophys Res Conn 27:523–530 www.ncbi.nlm.nih.gov/pubmed/5879460
Kirrolia A, Bishnoi NR, Singh N (2011) Salinity as a factor affecting the physiological and biochemical traits of Scenedesmus quadricauda. J Algal Biomass Utln 2:28–34
Lang I, Hodac L, Friedl T, Feussner I (2011) Fatty acid profiles and their distribution patterns in microalgae: A comprehensive analysis of more than 2000 strains from the SAG culture collection. BMC Plant Biol 124(11):1471–2229. doi:10.1186/1471-2229-11-124
Lepage, Roy (1986) Direct transesterfication of all classes of lipids in a one-step reaction. J Lipid Res 27:114–120 http://www.jlr.org/content/27/1/114.full.pdf
Levasseur M, Thompson P, Harrison PJ (1993) Physiological acclimation of marine phytoplankton to different nitrogen sources. J Phycol 29(5):587–595. doi:10.1111/j.0022-3646.1993.00587.x
Li Y, Chen YF, Chen P, Min M, Zhou W, Martinez B, Zhu J, Ruan R (2011) Characterization of a microalga Chlorella sp. well adapted to highly concentrated municipal wastewater for nutrient removal and biodiesel production. Bioresour Technol 102(08):5138–5144. doi:10.1016/j.biortech.2011.01.091
Li T, Zheng Y, Yu L, Chen S (2013) High productivity cultivation of a heat-resistant microalga Chlorella sorokiniana for biofuel production. Bioresour Technol 131:60–67. doi:10.1016/j.biortech.2012.11.121
Liu ZY, Wang GC, Zhou BC (2008) Effect of iron on growth and lipid accumulation in Chlorella Vulgaris. Bioresour Technol 99(11):4717–4722. doi:10.1016/j.biortech.2007.09.073
Ludwig TG, Goldberg VJH (1956) The anthrone method for the determination of carbohydrates in foods and in oral rinsing. J Dent Res 35:90–94. doi:10.1177/00220345560350012301
Lynn SG, Kilham SS, Kreeger DA, Interlandi SJ (2000) Effect of nutrient availability on the biochemical and elemental stoichiometry in freshwater diatom Stephanodiscus minutulus acillariophyceae. J Phycol 36(3):510–522
Mandotra SK, Kumar P, Suseela MR, Nayaka S, Ramteke PW (2016) Evaluation of fatty acid profile and biodiesel properties of microalga Scenedesmus abundans under the influence of phosphorus, pH and light intensities. Bioresour Technol 201:222–229. doi:10.1016/j.biortech.2015.11.042
Mandotra SK, Kumar P, Suseela MR, Ramteke PW (2014) Fresh water green microalga Scenedesmus abundans: a potential feedstock for high quality biodiesel production. Bioresour Technol 156:42–47. doi:10.1016/j.biortech.2013.12.127
McNeil SD, Nuccio ML, Hanson AD (1999) Betaines and related osmoprotectants. Targets for metabolic engineering of stress resistance. Plant Physiol 120(4):945–950. doi:10.1104/pp.120.4.945
Miao X, Wu Q (2007) Biodiesel production from heterotrophic microalgal oil. Bioresour Technol 97:841–846. doi:10.1016/j.biortech.2005.04.008
Moradi M, Ismail AM (2007) Responses of photosynthesis, chlorophyll fluorescence and ROS – Scavenging systems to salt stress. During seedling and reproductive stages of Rice Ann Botany 99:1161–1173. doi:10.1093/aob/mcm052
Mujtaba G, Choi W, Lee CG, Lee K (2012) Lipid production by Chlorella vulgaris after a shift from nutrient-rich to nitrogen starvation conditions. Bioresour Technol 123:279–283. doi:10.1016/j.biortech.2012.07.057
Na G, Lin Q, Li G, Tan Y, Huang L, Lin J (2012) Effect of salinity on growth, biochemical composition, and lipid productivity of Nannochloropsis oculata CS 179. Eng Life Sci 2012(12):1–7. doi:10.1002/elsc.201100204
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplast. Plant Cell Physiol 22(5):867–880. doi:10.1093/oxfordjournals.pcp.a076232
Ordog V, Stirk WA, Lovász C, Pulz O, Staden JV (2013) Lipid productivity and fatty acid composition in Chlorella and Scenepdesmus strains grown in nitrogen-stressed conditions. J Appl Phycol. doi:10.1007/s10811-012-9857-6
Pancha I, Chokshi K, Mishra S (2015) Enhanced biofuel production potential with nutritional stress amelioration through optimization of carbon source and light intensity in Scenedesmus sp. CCNM 1077. Bioresour Technol 179:565–572. doi:10.1016/j.biortech.2014.12.079
Patidar SK, Mitra M, George B, Soundarya R, Mishra S (2014) Potential of Monoraphidium minutum for carbon sequestration and lipid production in response to varying growth mode. Bioresour Technol 172:32–40
Patidar SK, Mitra M, Goel S, Mishra S (2016) Effect of carbon supply mode on biomass and lipid in CSMCRI's Chlorella variabilis (ATCC 12198). Biomass Bioenergy 86:1–10
Pereira H, Barreira L, Custódio L, Alrokayan S, Mouffouk F, Varela J, Abu-Salah KM, Hamadou RB (2013) Isolation and fatty acid profile of selected microalgae strains from the Red sea for biofuel production. Energies 6:2773–2783. doi:10.3390/en6062773
Rai AK, Abraham G (1993) Salinity tolerance and growth analysis of the cyanobacterium Anabaena doliolum. Bull Environ Contam. Toxicology 51(5):724–731
Ramos MJ, Fernandez CM, Casas A, Rodriguez L, Perez A (2009) Influence of fatty acid composition of raw materials on biodiesel properties. Bioresour Technol 100:261–268. doi:10.1016/j.biortech.2008.06.039
Rao AR, Dayananda C, Sarada R, Shamala TR, Ravishankar GA (2007) Effect of salinity on growth of green alga Botryococcus braunii and its constituents. Bioresour Technol 98:560–564. doi:10.1016/j.biortech.2006.02.007
Rodolfi L, Chini Zittelli G, Bassi N, Padovani G, Biondi N, Bonini G, Tredici MR (2009) Microalgae for oil: strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol Bioeng 102:100–112. doi:10.1002/bit.22033
Saha SK, Moane S, Murray P (2013) Effect of macro- and micro-nutrient limitation on superoxide dismutase activities and carotenoid levels in microalga Dunaliella salina CCAP 19/18. Bioresour Technol 147:23–28. doi:10.1016/j.biortech.2013.08.022
Salama ES, Kim CH, Reda AI, Shanab A, Kyu JM, Kwan OK, Seong HK, Byong HJ (2013) Biomass, lipid content, and fatty acid composition of freshwater Chlamydomonas mexicana and Scenedesmus obliquus grown under salt stress. Bioprocess Biosyst Eng 36:827–833. doi:10.1007/s00449-013-0919-1
Saumya D, Vibha D (2014) Effect of nitrogen concentration on lipid productivity and fatty acid composition of Monoraphidium sp. Bioresour Technol 152:572–575. doi:10.1016/j.biortech.2013.11.068
Shah MUS, Radziah CC, Ibrahim S, Latiff F, Othman MF, Abdullah MA (2013) Effects of photoperiod, salinity and pH on cell growth and lipid content of Pavlova lutheri. Ann Microbiol 64:157–164. doi:10.1007/s13213-013-0645-6
Shekh AY, Shrivastava P, Gupta A, Krishnamurthi K, Devi SS, Mudliar NS (2016) Biomass and lipid enhancement in chlorella sp. with emphasis on biodiesel quality assessment through detailed FAME signature. Bioresour Technol 201:276–286. doi:10.1016/j.biortech.2015.11.058
Shen FX, Chu FF, Lam KSP, Zeng RJ (2015) Biosynthesis of high yield fatty acids from Chlorella vulgaris NIES-227 under nitrogen starvation stress during heterotrophic cultivation. Water Res 81:294–300. doi:10.1016/j.watres.2015.06.003
Shih HH, Shu WH, Chun YC, Tomohisa H, Akihiko K, Jo-Shu C (2013) Characterization and optimization of carbohydrate production from an indigenous microalga Chlorella vulgaris FSP-E. Bioresour Technol 135:157–165. doi:10.1016/j.biortech.2012.10.100
Singh B, Guldhe A, Rawat I, Bux F (2014) Towards a sustainable approach for development of biodiesel from plant and microalgae. Renew Sust Energ Rev 29:216–245. doi:10.1016/j.rser.2013.08.067
Srivastava A, Singh SS, Mishra KA (2014) Modulation in fatty acid composition influences salinity stress tolerance in Frankia strains. Ann Microbiol 64:1315–1323. doi:10.1007/s13213-013-0775
Su CH, Chien LJ, Gomes J, Lin YS, Yu YK, Liou JS, Syu RJ (2011) Factors affecting lipid accumulation by Nannochloropsis oculata in a two-stage cultivation process. J Appl Phycol 23:903–908. doi:10.1007/s10811-010-9609-4
Sujatha K, Nagarajan P (2014) Effect of salinity on biomass and biochemical constituents of Spirulina platensis (Geitler). Internat J Plant Protec 7:71–73
Taguchi S, Hirata JA, Laws EA (1987) Silicate deficiency and lipid-synthesis of marine diatoms. J Phycol 23:260–267. doi:10.1111/j.1529-8817.1987.tb04133.x
Takagi M, Karseno, Yoshida T (2006) Effect of salt concentration on intracellular accumulation of lipids and triacylglyceride in marine microalgae Dunaliella cells. J Biosci Bioeng 101(3):223–226. doi:10.1263/jbb.101.223
Talebi AF, Mohtashami SK, Tabatabaei M, Tohidfar M, Bagheri A, Hadavand MH, Mirzajanzadeh M, Shafaroudi MS, Bakhtian S (2013) Fatty acids profiling: a selective criterion for screening microalgae strains for biodiesel production. Algal Res 2:258–267. doi:10.1016/j.algal.2013.04.003
Tomaselli L, Torzillo G, Giovanetti L, Bocci F, Tredici MR, Pusharaj B, Pupuazzo T, Balloni T, Meterassi R (1987) Recent research of Spirulina in Itali. Hydrobiol 151:79–82. doi:10.1007/978-94-009-4057-4_10
Vince O, Wendy AS, Peter B, Adeyemi OA, Ambrose O, Csaba L, Zoltán M, Johannes VS (2016) Effect of temperature and nitrogen concentration on lipid productivity and fatty acid composition in three Chlorella strains. Algal Res 16:141–149. doi:10.1016/j.algal.2016.03.001
Wang L, Li Y, Chen P, Min M, Chen Y, Zhu J, Ruan R (2010) Anaerobic digested dairy manure as a nutrient supplement for cultivation of oil-rich green microalgae Chlorella sp. Bioresour Technol 101(8):2623–2628. doi:10.1016/j.biortech.2009.10.062
Wang Y, He B, Sun Z, Chen FY (2016) Chemically enhanced lipid production from microalgae under low sub-optimal temperature. Algal Res 26–27. doi:10.1016/j.algal.2016.02.022
Wu YH, Yu Y, Li X, Hu HY, Su ZF (2012) Biomass production of a Scenedesmusn 804 sp. under phosphorous-starvation cultivation condition. Bioresourse Technology 112:193–198. doi:10.1016/j.biortech.2012.02.037
Xia L, Ge H, Zhou X, Zhang D, Hu C (2013) Photoautotrophic outdoor two-stage cultivation for oleaginous microalgae Scenedesmus Obtusus XJ-15. Bioresour Technol 144:261–267. doi:10.1016/j.biortech.2013.06.112
Xia L, Rong J, Yang H, He Q, Zhang D, Hu C (2014) NaCl as an effective inducer for lipid accumulation in freshwater microalgae Desmodesmus abundans. Bioresour Technol 161(0):402–409. doi:10.1016/j.biortech.2014.03.063
Xian S, Yu C, Hui X, Yan L, Jianrui S, Dairong Q, Yi C (2014) Effect of nitrogen-starvation, light intensity and iron on triacylglyceride/carbohydrate production and fatty acid profile of Neochloris oleoabundansHK-129 by a two-stage process. Bioresour Technol 155:204–212. doi:10.1016/j.biortech.2013.12.109
Xu XQ, Beardall J (1997) Effect of salinity on fatty acid composition of a green microalga from an Antarctic hypersaline lake. Phytochemistry 45(4):655–658. doi:10.1016/S0031-9422(96)00868-0
Yokoi S, Bressan RA, Hasegawa PM (2002) Salt stress tolerance of plants. JIRCAS Working Report 25–33
Yuqin L, Fangxin H, Hua X, Jinxiu M, Di C, Bo F, Hongyan Z (2014) Potential lipid accumulation and growth characteristic of the green alga Chlorella with combination cultivation mode of nitrogen (N) and phosphorus (P). Bioresour Technol 174:24–32. doi:10.1016/j.biortech.2014.09.142
Zhila NO, Kalacheva GS, Volova TG (2010) Effect of salinity on the biochemical composition of the alga Botryococcus braunii Kutz IPPAS H-252. J Appl Phycol 23:47–52. doi:10.1007/s10811-010-9532-8
Acknowledgements
The authors express sincere thanks to Dr. R.B.N Prasad (CSIR- IICT Hyderabad) for helping to analyze the samples for GC-MS and the Central University of Gujarat for providing the research facilities to carry out the present research study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Pandit, P.R., Fulekar, M.H. & Karuna, M.S.L. Effect of salinity stress on growth, lipid productivity, fatty acid composition, and biodiesel properties in Acutodesmus obliquus and Chlorella vulgaris . Environ Sci Pollut Res 24, 13437–13451 (2017). https://doi.org/10.1007/s11356-017-8875-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-8875-y