Abstract
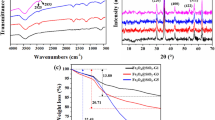
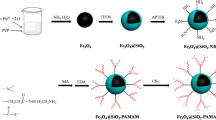
In this research, carboxyl-terminated hyperbranched poly(amidoamine) dendrimers grafted superparamagnetic nanoparticles (CT-HPMNPs) with core-shell structure were synthesized by the chemical co-precipitation method, the core of superparamagnetic iron oxide nanoparticles and a shell of polyamidoamines (PAMAM) and carboxyl groups, as a novel adsorbent for removing Hg2+ from aqueous systems. The surface of the particles was modified by 3-(aminopropyl) triethoxysilane, and finally, PAMAM and carboxyl dendrimers were grown on the surface up to 5.5 generation. The synthesized polymer was characterized physically and morphologically using different techniques. Also, they were evaluated in terms of adsorption capacity to remove inorganic pollutants of Hg2+, selectivity, and reusability. The adsorption mechanism Hg2+ onto CT-HPMNPs was investigated by single-step and two-step isotherms that the adsorption capacity of Hg2+ obtained 72.3 and 32.88 mg g−1 respectively at pH 5, adsorbent dosage 2 g L−1, Hg2+ initial concentrations 20 mg L−1, contact time 60 min, and temperature of 298 K by CT-HPMNPs. Also, the kinetics of Hg2+ followed the pseudo-second-order model and adsorption isotherms of Hg2+ onto CT-HPMNPs were fitted well by Freundlich (as a single-step) and two-step adsorption models with a correlation coefficient of 0.9997 and 0.9999 respectively. The results showed a significant potential of Hg2+ ions removing from industrial wastewater and spiked water by CT-HPMNPs.















Similar content being viewed by others
References
Aghdam K, Panahi HA, Alaei E et al (2016) Preparation of functionalized graphene oxide and its application as a nanoadsorbent for Hg2+ removal from aqueous solution. Environ Monit Assess 188:223
Alizadeh T, Nayeri S (2018) Electrocatalytic oxidation of salicylic acid at a carbon paste electrode impregnated with cerium-doped zirconium oxide nanoparticles as a new sensing approach for salicylic acid determination. J Solid State Electrochem 22:2039–2048
Alizadeh T, Hamidi N, Ganjali MR, Nourozi P (2017) Development of a highly selective and sensitive electrochemical sensor for Bi3+ determination based on nano-structured bismuth-imprinted polymer modified carbon/carbon nanotube paste electrode. Sensors Actuators B Chem 245:605–614
Anjum M, Miandad R, Waqas M et al (2019) Remediation of wastewater using various nanomaterials. Arab J Chem 12(8):4897–4919. https://doi.org/10.1016/j.arabjc.2016.10.004
Asiabi H, Yamini Y, Shamsayei M, Molaei K, Shamsipur M (2018) Functionalized layered double hydroxide with nitrogen and sulfur co-decorated carbondots for highly selective and efficient removal of soft Hg2+ and Ag+ ions. J Hazard Mater 357:217–225
Aslani H, Kosari TE, Naseri S et al (2018) Hexavalent chromium removal from aqueous solution using functionalized chitosan as a novel nano-adsorbent: modeling and optimization, kinetic, isotherm, and thermodynamic studies, and toxicity testing. Environ Sci Pollut Res 25:20154–20168
Babaei AA, Alaee Z, Ahmadpour E, Ramazanpour-Esfahani A (2014) Kinetic modeling of methylene blue adsorption onto acid-activated spent tea: a comparison between linear and non-linear regression analysis. J Adv Environ Health Res 2:197–208
Baharin S, Muhamad Sarih N, Mohamad S (2016) Novel functionalized polythiophene-coated Fe3O4 nanoparticles for magnetic solid-phase extraction of phthalates. Polymers (Basel) 8:117
Baniasadi M, Tajabadi M, Nourbakhsh MS, Kamali M (2014) Synthesis and characterization of CORE-shell nanostructure containing super paramagnetic magnetite and poly (Amidoamine)(Pamam) dendrimers
Bao J, Fu Y, Bao Z (2013) Thiol-functionalized magnetite/graphene oxide hybrid as a reusable adsorbent for Hg 2+ removal. Nanoscale Res Lett 8:486
Blue LY, Jana P, Atwood DA (2010) Aqueous mercury precipitation with the synthetic dithiolate , BDTH 2. Fuel 89:1326–1330. https://doi.org/10.1016/j.fuel.2009.10.031
Can M (2015) Studies of the kinetics for rhodium adsorption onto gallic acid derived polymer: the application of nonlinear regression analysis. System 10:11
Canada H (2012) Guidelines for Canadian drinking water quality—summary table. Water, air Clim. Chang. Bur. Heal. Environ. Consum. Saf. Branch
Chen X (2015) Modeling of experimental adsorption isotherm data. Information 6:14–22
Chu TPM, Nguyen NT, Vu TL et al (2019) Synthesis, characterization, and modification of alumina nanoparticles for cationic dye removal. Materials (Basel) 12:450
Dave PN, Chopda LV (2014) Application of iron oxide nanomaterials for the removal of heavy metals. J Nanotechnol 2014
Edition F (2011) Guidelines for drinking-water quality. WHO Chron 38:104–108
Ejikeme PCN, Ejikeme EM, Okonkwo GN (2014) Equilibrium, kinetic and thermodynamic studies on basic dye adsorption using composite activated carbon. Int J Tech Res Appl 2:96–103
Fan Y-H, Zhang S-W, Qin S-B et al (2018) An enhanced adsorption of organic dyes onto NH2 functionalization titanium-based metal-organic frameworks and the mechanism investigation. Microporous Mesoporous Mater 263:120–127
Farrukh A, Akram A, Ghaffar A, Hanif S, Hamid A, Duran H, Yameen B (2013) Design of polymer-brush-grafted magnetic nanoparticles for highly efficient water remediation. ACS Appl Mater Interfaces 5:3784–3793
Figueira P, Lopes CB, Daniel-da-Silva AL, Pereira E, Duarte AC, Trindade T (2011) Removal of mercury (II) by dithiocarbamate surface functionalized magnetite particles: application to synthetic and natural spiked waters. Water Res 45:5773–5784
Ghaffari HR, Pasalari H, Tajvar A et al (2017) Linear and nonlinear two-parameter adsorption isotherm modeling: a case-study. Int J Eng Sci 6:1–11
Ghosh S, Maji S, Mondal A (2018) Study of selective sensing of Hg2+ ions by green synthesized silver nanoparticles suppressing the effect of Fe3+ ions. Colloids Surf A Physicochem Eng Asp 555:324–331
Hadavifar M, Bahramifar N, Younesi H, Li Q (2014) Adsorption of mercury ions from synthetic and real wastewater aqueous solution by functionalized multi-walled carbon nanotube with both amino and thiolated groups. Chem Eng J 237:217–228. https://doi.org/10.1016/j.cej.2013.10.014
Hakami O, Zhang Y, Banks CJ (2012) Thiol-functionalised mesoporous silica-coated magnetite nanoparticles for high efficiency removal and recovery of Hg from water. Water Res 46:3913–3922
Homayonfard A, Miralinaghi M, Shirazi RHSM, Moniri E (2018) Efficient removal of cadmium (II) ions from aqueous solution by CoFe2O4/chitosan and NiFe2O4/chitosan composites as adsorbents. Water Sci Technol 78:2297–2307
Hossien M, Nodoushan S, Parvizi Z, Nodoushan FM (2017) Adsorption of arsenite from aqueous solutions using granola modified lemon peel 4. https://doi.org/10.5812/ajehe.11667.Research
Kazemi A, Bahramifar N, Heydari A, Olsen SI (2019) Synthesis and sustainable assessment of thiol-functionalization of magnetic graphene oxide and superparamagnetic Fe3O4@ SiO2 for Hg (II) removal from aqueous solution and petrochemical wastewater. J Taiwan Inst Chem Eng 95:78–93
Khazaei M, Nasseri S, Ganjali MR et al (2018) Selective removal of mercury (II) from water using a 2, 2-dithiodisalicylic acid-functionalized graphene oxide nanocomposite: kinetic, thermodynamic, and reusability studies. J Mol Liq 265:189–198
Khodadust R, Unsoy G, Yalcın S et al (2013) PAMAM dendrimer-coated iron oxide nanoparticles: synthesis and characterization of different generations. J Nanopart Res 15:1488
Li J, Li X, Alsaedi A et al (2018) Synthesis of highly porous inorganic adsorbents derived from metal-organic frameworks and their application inefficient elimination of mercury (II). J Colloid Interface Sci. https://doi.org/10.1016/j.jcis.2018.01.112
Li Y, Xia M, An F, Ma N, Jiang X, Zhu S, Wang D, Ma J (2019) Superior removal of Hg (II) ions from wastewater using hierarchically porous, functionalized carbon. J Hazard Mater 371:33–41
Liu Y, Li M, He C (2017) Removal of Cr (VI) and Hg (II) ions from wastewater by novel β-CD/MGO-SO3H composite. Colloids Surfaces A Physicochem Eng Asp 512:129–136
Mandel K, Hutter F, Gellermann C, Sextl G (2012) Modified superparamagnetic nanocomposite microparticles for highly selective HgII or CuII separation and recovery from aqueous solutions. ACS Appl Mater Interfaces 4:5633–5642
Nethaji S, Sivasamy A, Mandal AB (2013) Adsorption isotherms, kinetics and mechanism for the adsorption of cationic and anionic dyes onto carbonaceous particles prepared from Juglans regia shell biomass. Int J Environ Sci Technol 10:231–242
Panahi HA, Tavanaei Y, Moniri E, Keshmirizadeh E (2014) Synthesis and characterization of poly [N-isopropylacrylamide-co-1-(N, N-bis-carboxymethyl) amino-3-allylglycerol] grafted to magnetic nano-particles for the extraction and determination of fluvoxamine in biological and pharmaceutical samples. J Chromatogr A 1345:37–42
Parham H, Zargar B, Shiralipour R (2012) Fast and efficient removal of mercury from water samples using magnetic iron oxide nanoparticles modified with 2-mercaptobenzothiazole. J Hazard Mater 205:94–100
Pham TD, Do DTT, Ha AVL, et al (2017) Adsorptive removal of ammonium ion from aqueous solution using surfactant-modified alumina. pp 327–337
Pham T, Bui T, Nguyen V et al (2018a) Adsorption of polyelectrolyte onto nanosilica synthesized from rice husk: characteristics, mechanisms, and application for antibiotic removal. Polymers (Basel) 10:220
Pham TD, Do TU, Pham TT, et al (2018b) Adsorption of poly ( styrenesulfonate ) onto different-sized alumina particles : characteristics and mechanisms
Pham TD, Tran TT, Pham TT et al (2019) Adsorption characteristics of molecular oxytetracycline onto alumina particles: the role of surface modification with an anionic surfactant. J Mol Liq 287:110900
Santhosh C, Daneshvar E, Kollu P et al (2017) Magnetic SiO2@ CoFe2O4 nanoparticles decorated on graphene oxide as efficient adsorbents for the removal of anionic pollutants from water. Chem Eng J 322:472–487
Shafizadeh F, Taghizadeh M, Hassanpour S (2019) Preparation of a novel magnetic Pd (II) ion-imprinted polymer for the fast and selective adsorption of palladium ions from aqueous solutions. Environ Sci Pollut Res:1–16
Shan C, Ma Z, Tong M, Ni J (2015) Removal of Hg (II) by poly (1-vinylimidazole)-grafted Fe3O4@ SiO2 magnetic nanoparticles. Water Res 69:252–260
Sun Q, Aguila B, Perman J, et al (2017) Postsynthetically Modi fi ed covalent organic frameworks for. https://doi.org/10.1021/jacs.6b12885
Thekkudan VN, Vaidyanathan VK, Ponnusamy SK et al (2016) Review on nanoadsorbents: a solution for heavy metal removal from wastewater. IET Nanobiotechnol 11:213–224
Tizro S, Baseri H (2016) Heavy metals removal from wastewater by using different kinds of magnetite nano-adsorbents: effects of different organic and inorganic coatings on the removal of copper and lead ions. J Adv Mat Process 4:15–29
Tran HN, Chao H-P (2018) Adsorption and desorption of potentially toxic metals on modified biosorbents through new green grafting process. Environ Sci Pollut Res 25:12808–12820
Wang Z, Wu D, Wu G et al (2013) Modifying Fe3O4 microspheres with rhodamine hydrazide for selective detection and removal of Hg2+ ion in water. J Hazard Mater 244:621–627
Wang L, Zhao X, Zhang J, Xiong Z (2017) Selective adsorption of Pb (II) over the zinc-based MOFs in aqueous solution-kinetics, isotherms, and the ion exchange mechanism. Environ Sci Pollut Res 24:14198–14206
Yue X, Guo W, Li X, Zhou H, Wang R (2016) Core-shell Fe3O4@MIL-101 (Fe) composites as heterogeneous catalysts of persulfate activation for the removal of acid orange 7. Environ Sci Pollut Res 23:15218–15226
Zhang C, Sui J, Li J et al (2012) Efficient removal of heavy metal ions by thiol-functionalized superparamagnetic carbon nanotubes. Chem Eng J 210:45–52
Zhang Y, Yan L, Xu W et al (2014) Adsorption of Pb (II) and Hg (II) from aqueous solution using magnetic CoFe2O4-reduced graphene oxide. J Mol Liq 191:177–182
Zhu B, Zhao J, Yu H et al (2013) Naphthalimide-functionalized Fe3O4@SiO2 core/shell nanoparticles for selective and sensitive adsorption and detection of Hg2+. Chem Eng J 219:411–418
Zou C, Jiang W, Liang J et al (2019) Removal of Pb (II) from aqueous solutions by adsorption on magnetic bentonite. Environ Sci Pollut Res 26:1315–1322
Acknowledgments
The authors gratefully acknowledge the support of the Department of Natural Resources and Environment, Science and Research Branch, Islamic Azad University, Tehran, Iran.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Additional information
Responsible Editor: Tito Roberto Cadaval Jr
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tabatabaiee Bafrooee, A.A., Ahmad Panahi, H., Moniri, E. et al. Removal of Hg2+ by carboxyl-terminated hyperbranched poly(amidoamine) dendrimers grafted superparamagnetic nanoparticles as an efficient adsorbent. Environ Sci Pollut Res 27, 9547–9567 (2020). https://doi.org/10.1007/s11356-019-07377-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-07377-z