Abstract
Purpose
Factors such as organic matter can significantly influence the distribution of mercury (Hg) in aquatic environments. Recent studies in Arctic and sub-Arctic lakes in Canada have investigated whether scavenging of Hg by phytoplankton significantly affects distributions of Hg in sediments. This study examined the relationships between Hg and organic components in two contrasting lakes (Lakes Qinghai and Chenghai) in low and middle latitudes of China.
Materials and methods
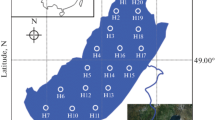
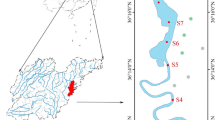
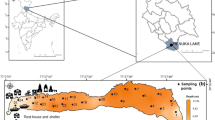
Sediment cores from the less-polluted, oligotrophic Lake Qinghai (QH) and from the polluted, eutrophic Lake Chenghai (CH) were collected by a gravity corer. The cores were sectioned and transported on ice to the laboratory where they were stored at −20 °C. Subsamples were dried in a vacuum freeze dryer and grounded with a mortar and pestle prior to analyses. Total concentrations of Hg were quantified using cold vapor atomic absorption spectrometry. Total organic carbon (TOC) was quantified using an elemental analyzer after removal of carbonate. The Rock-Eval 6 pyrolysis technique (Vinci Technologies, Rueil-Malmaison, France) was used to deconvolute TOC in sediments into S1, S2, and RC components; S2 was further separated into S2a and S2b.
Results and discussion
Different relationships between Hg and TOC were found in the two lakes, which suggest that different types of organic compounds might play completely different roles in the distribution of Hg in lakes. S1 (the soluble organic matter (SOM)) was found to significantly control distributions of Hg in sediments of both lakes, while S2 and S2a were not. Combining the synchronous fluctuations of Hg and the oxygen index in the QH sediment core and in recent sections of CH suggested that allochthonous SOM derived from the terrestrial environment had an important influence on the distribution of Hg in both lakes and a large portion of Hg that originated from the lake catchment.
Conclusions
This study provides further evidence that organic matter is one of the most important factors that influences distributions of Hg in lake sediments and that SOM was the primary form of carbon associated with sedimentation of Hg. The results also suggest that Hg in lake sediments might not accurately represent its pollution history as it could also be influenced by land use, such as agriculture or other human activities in the catchment.






Similar content being viewed by others
References
Bai YC, Wu FC, Liu CQ, Li W, Guo JY, Fu PQ, Xing BS, Zheng J (2008) Ultraviolet absorbance titration for determining stability constants of humic substances with Cu(II) and Hg(II). Anal Chim Acta 616:115–121
Carrie J, Sanei H, Goodarzi F, Stern G, Wang F (2009) Characterization of organic matter in surface sediments of the Mackenzie River Basin, Canada. Int J Coal Geol 77:416–423
Carrie J, Sanei H, Stern G (2012) Standardisation of Rock-Eval pyrolysis for the analysis of recent sediments and soils. Org Geochem 46:38–53
Cooke CA, Wolfe AP, Michelutti N, Balcom PH, Briner JP (2012) A Holocene perspective on algal mercury scavenging to sediments of an arctic lake. Environ Sci Tech 46:7135–7141
Deison R, Smol JP, Kokelj SV, Pisaric MFJ, Kimpe LE, Poulain AJ, Sanei H, Thienpont JR, Blais JM (2012) Spatial and temporal assessment of mercury and organic matter in thermokarst affected lakes of the Mackenzie Delta Uplands, NT, Canada. Environ Sci Tech 46(16):8748–55
Drexel RT, Haitzer M, Ryan JN, Aiken GR, Nagy KL (2002) Mercury(II) sorption to two Florida everglades peats: evidence for strong and weak binding and competition by dissolved organic matter released from the peat. Environ Sci Technol 36:4058–4064
Fu PQ, Wu FC, Liu CQ, Wang FY, Li W, Yue LX, Guo QJ (2007) Fluorescence characterization of dissolved organic matter in an urban river and its complexation with Hg(II). Appl Geochem 22:1668–1679
Haitzer M, Aiken GR, Ryan JN (2002) Binding of mercury(II) to dissolved organic matter: the role of the mercury-to-DOM concentration ratio. Environ Sci Technol 3:3564–3570
Joensuu OI (1971) Fossil fuels as a source of mercury pollution. Science 172:1027–1028
Johannesson LJ, Stevens RS, Eriksson KE (2003) The influence of an urban stream on sediment geochemistry in Göteborg Harbour, Sweden. Environ Geol 43:434–444
Kainz M, Lucotte M, Parrish CC (2003) Relationships between organic matter composition and methyl mercury content of offshore and carbon-rich littoral sediments in an oligotrophic lake. Can J Fish Aquat Sci 60:888–896
Kainz M, Lucotte M (2006) Mercury concentrations in lake sediments—revisiting the predictive power of catchment morphometry and organic matter composition. Water Air Soil Pollut 170:173–189
Kirk JL, Muir DCG, Antoniades D, Douglas MSV, Evans MS, Jackson TA, Kling H, Lamoureux S, Lim DSS, Pienitz R, Smol JP, Stewart K, Wang X, Yang F (2011) Response to comment on climate change and mercury accumulation in Canadian high and subarctic lakes. Environ Sci Technol 45:6705–6706
Kirk JL, Muir DCM, Antoniades D, Douglas MSV, Evans MS, Jackson TA, Kling H, Lamoureux S, Lim DSS, Pienitz R, Smol JP, Stewart K, Wang X, Yang F (2012) Climate change and mercury accumulation in Canadian high and subarctic lakes. Environ Sci Technol 45:964–970
Lafargue E, Marquis F, Pillot D (1998) Rock-Eval 6 applications in hydrocarbon exploration, production, and soil contamination studies. Revue d’IFP 53:421–437
Lindberg SE, Harriss RC (1974) Mercury–organic matter associations in estuarine sediments and interstitial water. Environ Sci Technol 8:459–462
Loux NT (1998) An assessment of mercury-species-dependent binding with natural organic carbon. Chem Spec Bioavail 10:127–136
Marvin-DiPasquale M, Lutz MA, Brigham ME, Krabbenhoft DP, Aiken GR, Orem WH, Hall BD (2009) Mercury cycling in stream ecosystems. 2. Benthic methylmercury production and bed sediment-pore water partitioning. Environ Sci Technol 43:2726–2732
Mirlean N, Andrus VE, Baisch P (2003) Mercury pollution sources in sediments of Patos Lagoon Estuary, Southern Brazil. Mar Pollut Bull 46:331–334
Outridge PM, Sanei H, Stern GA, Hamilton PB, Goodarzi F (2007) Evidence for control of mercury accumulation rates in Canadian high Arctic lake sediments by variations of aquatic primary productivity. Environ Sci Technol 41:5259–5265
Outridge PM, Sanei H, Stern GA, Goodsite M, Hamilton PB, Carrie J, Goodarzi F, Macdonald RW (2011) Comment on climate change and mercury accumulation in Canadian high and subarctic lakes. Environ Sci Technol 45:6703–6704
Parsons MJ, Long DT, Yohn SS, Giesy JP (2007) Spatial and temporal trends of mercury loadings to Michigan inland lakes. Environ Sci Technol 41:5634–5640
Poissant L (1999) Potential sources of atmospheric total gaseous mercury in the St. Lawrence River valley. Atmos Environ 33:2537–2547
Ravichandran M (2004) Interactions between mercury and dissolved organic matter—a review. Chemosphere 55:319–331
Regnell O, Ewald G, Lord E (1997) Factors controlling temporal variation in methyl mercury levels in sediment and water in a seasonally stratified lake. Limnol Oceanogr 42:1784–1795
Seigneur C, Vijayaraghavan K, Lohman K, Karamchandani P, Scott C (2003) Global source attribution for mercury deposition in the United States. Environ Sci Technol 38:555–569
Sanei H, Stasiuk LD, Goodarzi F (2005) Petrological changes occurring in organic matter from recent lacustrine sediments during thermal alteration by Rock-Eval pyrolysis. Org Geochem 36:1190–1203
Sanei H, Goodarzi F (2006) Relationship between organic matter and mercury in recent lake sediment: The physical-geochemical aspects. Appl Geochem 21:1900–1912
Stern GA, Sanei H, Roach P, DeLaronde J, Outridge PM (2009) Historical interrelated variations of mercury and aquatic organic matter in lake sediment cores from a subArctic lake in Yukon, Canada: further evidence toward the algal-mercury scavenging hypothesis. Environ Sci Technol 43:7684–7690
Wallschläger D, Desai MVM, Wilken R-D (1996) The role of humic substances in the aqueous mobilization of mercury from contaminated floodplain soils. Water Air Soil Pollut 90:507–520
Wan GJ, Chen JA, Wu FC, Xu SQ, Bai ZG, Wan EY, Wang CS, Huang RG, Yeager KM, Santschi PH (2005) Coupling between 210Pbex and organic matter in sediments of a nutrient-enriched lake: an example from Lake Chenghai, China. Chem Geol 224:223–236
Wu F, Cai Y, Evans D, Dillon P (2004) Complexation between Hg(II) and dissolved organic matter in stream waters: and application of fluorescence spectroscopy. Biogeochemistry 71:339–351
Wu F, Xu L, Sun Y, Liao H, Zhao X, Guo J (2012) Exploring the relationship between polycyclic aromatic hydrocarbons and sedimentary organic carbon in three Chinese lakes. J Soils Sediments 12:774–783
Xia K, Skyllberg UL, Bleam WF, Bloom PR, Nater EA, Helmke PA (1999) X-ray absorption spectroscopic evidence for the complexation of Hg(II) by reduced sulfur in soil humic substances. Environ Sci Technol 33:257–261
Xu L, Wu F, Zheng J, Xie Q, Li H, Liao H, Zhao X, Guo F (2011a) Sediment records of Sb and Pb stable isotopic ratios in Lake Qinghai. Microchem J 97:25–29
Xu L, Wu F, Wan G, Liao H, Zhao X, Xing B (2011b) Relationship between 210Pbex activity and sedimentary organic carbon in sediments of 3 Chinese lakes. Environ Pollut 159:3462–3467
Yang JX, Qi HF, Shi JQ, Yao WZ (2008) Summer investigation of aquatic organism in Lake Qinghai. Qinghai Science and Technology 6:19–25 (in Chinese)
Acknowledgments
The research was supported by the National Natural Science Foundation of China (41003048, 40973090 and 41261140337).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Jay Gan
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
(DOC 28 kb)
Rights and permissions
About this article
Cite this article
Wu, F., Xu, L., Liao, H. et al. Relationship between mercury and organic carbon in sediment cores from Lakes Qinghai and Chenghai, China. J Soils Sediments 13, 1084–1092 (2013). https://doi.org/10.1007/s11368-013-0694-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-013-0694-2