Abstract
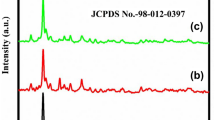
Nanocrystalline Li2TiO3 was successfully synthesized using solid-state reaction method. The microstructural and electrochemical properties of the prepared material are systematically characterized. The X-ray diffraction pattern of the prepared material exhibits predominant (002) orientation related to the monoclinic structure with C2/c space group. HRTEM images and SAED analysis reveal the well-developed nanostructured particles with average size of ∼40 nm. The electrochemical properties of the prepared sample are carried out using cyclic voltammetry (CV) and chronopotentiometry (CP) using Pt//Li2TiO3 cell in 1 mol L−1 Li2SO4 aqueous electrolyte. The Li2TiO3 electrode exhibits a specific discharge capacity of 122 mAh g−1; it can be used as anode in Li battery within the potential window 0.0–1.0 V, while investigated as a supercapacitor electrode, it delivers a specific capacitance of 317 F g−1 at a current density of 1 mA g−1 within the potential range −0.4 to +0.4 V. The demonstration of both anodic and supercapacitor behavior concludes that the nanocrystalline Li2TiO3 is a suitable electrode material for supercapattery application.














Similar content being viewed by others
References
Lee SH, Lee SG, Yoon JR, Kim HK (2015) Novel performance of ultrathin AlPO4 coated H2Ti12O25 exceeding Li4Ti5O12 in cylindrical hybrid supercapacitor. J Power Sources 273:839–843
Chen C, Xu G, Wei X, Yang L (2016) A macroscopic three-dimensional tetrapod-separated graphene-like oxygenated N-doped carbon nanosheet architecture for use in supercapacitors. J Mater Chem A 4:9900–9909
Maiti S, Mahanty S (2015) Influence of imidazolium-based ionic liquid electrolytes on the performance of nano-structured MnO2 hollow spheres as electrochemical supercapacitor. RSC Adv 5:41617–41626
Zheng X, Gu ZX, Hu Q, Geng B, Zhang X (2015) Ultrathin porous nickel-cobalt hydroxide nanosheets for high performance supercapacitor electrodes. RSC Adv 5:17007–17013
Zhao C, Zheng W (2015) A review for aqueous electrochemical supercapacitors. Front Energy Res 3:23
Julien CM, Mauger A, Vijh A, Zaghib K (2016) Lithium batteries: science and technology. Springer, Heidelberg
Stevenson AJ, Gromadskyi DG, Hu D, Chae J, Guan L, Yu L, Chen GZ (2015) Supercapatteries with hybrids of redox active polymers and nanostructured carbons. In: Nanocarbons for advanced energy storage. Wiley-VCH Verlag GmbH & Co KGaA, Desden, pp 179–210
Yu L, George Z (2016) Redox electrode materials for supercapatteries. J Power Sources 326:604–612
Lindstrom H, Sodergren S, Solbrand A, Rensmo H, Hjelm J, Hagfeldt A, Lindquist E (1997) Li+ ion insertion in TiO2 (anatase). 2. Voltametry on nanoporous films. J Phys Chem B 101:7717–7722
Fattakhova D, Kavan L, Krtil P (2001) Lithium insertion into titanium dioxide (anatase) electrodes: microstructure and electrolyte effects. J Solid State Electrochem 5:196–204
Kavan L (2014) Lithium insertion into TiO2 (anatase): electrochemistry, Raman spectroscopy, and isotope labeling. J Solid State Electrochem 18:2297–2306
Dylla AG, Henkelman G, Stevenson KJ (2013) Lithium insertion in nanostructured TiO2(B) architectures. Acc Chem Res 46:1104–1112
Kavan L, Fattakhova D, Krtil P (1999) Lithium insertion into mesoscopic and single-crystal TiO2 (rutile). J Electrochem Soc 146:915–919
Hu YS, Kienle L, Guo YG, Maier J (2006) High lithium electroactivity of nanometer-sized rutile TiO2. Adv Mater 18:1421–1426
Kuhn A, Amandi R, Garcia-Alvarado F (2001) Electrochemical lithium insertion in TiO2 with the ramsdellite structure. J Power Sources 92:221–227
Colbow KM, Dahn JR, Haering RR (1989) Structure and electrochemistry of the spinel oxides LiTi2O4 and Li4/3Ti5/3O4. J Power Sources 26:397–402
Zeng ZY, Tu JP, Wang XL, Zhao XB (2008) Electrochemical properties of Si/LiTi2O4 nanocomposite as anode materials for Li-ion secondary batteries. J Electroanal Chem 616:7–13
Zaghib K, Simoneau M, Armand M, Gauthier M (1999) Electrochemical study of Li4Ti5O12 as negative electrode for Li-ion polymer rechargeable batteries. J Power Sources 81:300–305
Bohnke C, Fourquet JL, Randrianantoandro N, Brousse T, Crosnier O (2002) Electrochemical insertion of lithium into the ramdellite-type oxide Li2Ti3O7: influence of the Li2Ti3O7 particle size. J Solid State Electrochem 6:403–411
Kleykamp H (2002) Phase equilibria in the li-Ti-O system and physical properties of Li2TiO3. Fusion Eng Des 61-62:361–366
Islam MM, Bredow T (2016) Lithium diffusion pathways in β-Li2TiO3: a theoretical study. J Phys Chem C 120:7061–7066
Yu CL, Wang F, Cao SY, Gao DP, Hui HB, Guo YY, Wang DY (2015) The structure of H2TiO3—a short discussion on lithium recovery from salt lake brine by H2TiO3. Dalton Trans 44:15721–15724
Lu J, Peng Q, Wang W, Nan C, Li L, Li Y (2013) Nanoscale coating of LiMO2 (M = Ni, Co, Mn) nanobelts with Li+-conductive Li2TiO3: toward better rate capabilities for Li-ion batteries. J Am Chem Soc 135:1649–1652
Shi J, Liang Y, Li L, Peng Y, Yang H (2015) Evaluation of the electrochemical characteristics of silicon/lithium titanate composite as anode material for lithium ion batteries. Electrochim Acta 155:125–131
Peng L, Zhang H, Fang L, Zhang Y, Wang Y (2016) Novel peapoded Li4Ti5O12 nanoparticles for high-rate and ultralong-life rechargeable lithium ion batteries at room and lower temperatures. Nanoscale 8:2030–2040
Wang Y, Zhou A, Dai X, Feng L, Li J (2014) Solid-state synthesis of submicron-sized Li4Ti5O12/Li2TiO3 composites with rich grain boundaries for lithium ion batteries. J Power Sources 266:114–120
Yu CL, Yanagisawa K, Kamiya S, Kozawa T, Ueda T (2014) Monoclinic Li2TiO3 nano-particles via hydrothermal reaction: processing and structure. Ceram Int 40:1901–1908
Yu CL, Gao DP, Yanagisawa K (2014) Vacancy and substitution defects of β-Li2TiO3 prepared by hydrothermal method. Chem Lett 43:369–370
Wu X, Wen Z, Lin B, Xu X (2008) Sol–gel synthesis and sintering of nano-size Li2TiO3 powder. Mater Lett 62:837–839
Yu CL, Wang F, Zhang AL, Gao DP, Cao SY, Guo YY, Hui HB, Hao X, Wang DY, Yanagisawa K (2015) Preparation of β-Li2TiO3 pebbles by a modified indirect wet chemistry method. Fusion Eng Des 101:73–79
Zhou Q, Gao Y, Mou Y, Xue L, Li H, Youwei YY (2014) Rapid fabrication of Li2TiO3 pebbles via microwave-induced combustion process and 3D printing. Proc. of the 22nd Inter. Conf. on Nuclear Engineering. doi:10.1115/ICONE22-30394
Lee MH, Jung H, Lee SJ (2007) Highly sinterable lithium titanate powders fabricated by an organic-inorganic solution route. Solid State Phenom (Switzerland) 124-126:807–810
Kataoka K, Takahashi Y, Kijima N, Nagai H, Akimoto J, Idemoto Y, Ohshima KI (2009) Crystal growth and structure refinement of monoclinic Li2TiO3. Mater Res Bull 44:168–172
Ramaraghavulu R, Buddhudu S, Bhaskar-Kumar G (2011) Analysis of structural and thermal properties of Li2TiO3 ceramic powders. Ceram Int 37:1245–1249
Bian JJ, Dong YF (2011) Sintering behavior, microstructure and microwave dielectric properties of Li2+x TiO3 (0 ≤ x ≤ 0.2). Mater Sci Eng B 176:147–151
Chauvaut V, Cassir M (1999) Electrochemical intercalation of Li+ in Li2TiO3 at 600 and 650 °C. J Electroanal Chem 474:9–15
Tabuchi M, Nakashima A, Shigemura H, Ado K, Kobayashi H, Sakaebe H, Tatsumi K, Kageyama H, Nakamura T, Kanno R (2003) Fine Li(4-x)/3Ti(2-2x)/3FexO2 (0.18≤x≤0.67) powder with cubic rock-salt structure as a positive electrode material for rechargeable lithium batteries. J Mater Chem 13:1747–1757
Morales J, Santos-Pena J, Trocoli R, Franger S (2008) Electrochemical activity of rock-salt-structured LiFeO2-Li4/3Ti2/3O2 nanocomposites in lithium cells. J Nanopart Res 10:217–226
Shigemura H, Tabuchi M, Sakaebe H, Kobayashi H, Kageyama H (2003) Lithium extraction and insertion behavior of nanocrystalline Li2TiO3-LiFeO2 solid solution with cubic rock salt structure. J Electrochem Soc 150:A638–A644
Zhang L, Wang X, Noguchi H, Yoshio M, Takada K, Sasaki T (2004) Electrochemical and ex situ XRD investigations on (1-x)LiNiO2•xLi2TiO3 (0.05x0.5). Electrochim Acta 49:3305–3311
Bhatti HS, Anjum DH, Ullah S, Ahmed B, Habib A, Karim A, Hasanain SK (2016) Electrochemical characteristics and Li+ ion intercalation kinetics of dual-phase Li4Ti5O12/Li2TiO3 composite in the voltage range 0−3 V. J Phys Chem C 120:9553–9561
Rosaiah P, Hussain OM (2013) Synthesis, electrical and dielectrical properties of lithium iron oxide. Adv Mat Lett 4:288–295
Dorrian JF, Newnham RE (1969) Refinement of the structure of Li2TiO3. Mater Res Bull 4:179–184
Denisova TA, Maksimova LG, Polyakov EV, Zhuravlev NA, Kovyazina SA, Leonidova ON, Khabibulin DF, Yur’eva EI (2006) Metatitanic acid: synthesis and properties. Russ J Inorg Chem 51:691–699
Nakazawa T, Naito A, Aruga T, Grismanovs V, Chimi Y, Iwasz A, Jitsukawa S (2007) High energy heavy ion induced structural disorder in Li2TiO3. J Nucl Mater 367-370:1398–1403
Krtil P, Fattakhova D (2011) Li insertion into li-Ti-O spinels: voltammetric and electrochemical impedance spectroscopy study. J Electrochem Soc 148:A1045–A1050
Dash U, Sahoo S, Chaudhuri P, Parashar SKS, Parashar K (2014) Electrical properties of bulk and nano Li2TiO3 ceramics: a comparative study. J Adv Ceram 3:89–97
Hao YZ, Zhang QL, Zhang J, Xin CR, Yang H (2012) Enhanced sintering characteristics and microwave dielectric properties of Li2TiO3 due to nano-size and non-stoichiometry effect. J Mater Chem 22:23885–23892
Monchak M, Dolotko O, Muhlbauer MJ, Baran V, Senyshyn A, Ehrenberg H (2016) Monoclinic β-Li2TiO3: neutron diffraction study and estimation of Li diffusion pathways. Solid State Sci 61:161–166
Ganesan M, Dhananjeyan MVT, Sarangapani KB, Renganathan NG (2007) Solid state rapid quenching method to synthesize micron size Li4Ti5O12. J Electroceram 18:329–337
Signorelli R, Ku DC, Kassakian JG, Schindall JE (2009) Electrochemical double-layer capacitors using carbon nanotube electrode structures. Proc IEEE 97:1837–1847
Ansari SA, Parveen N, Han TH, Ansari MO, Cho MH (2016) Fibrous polyaniline@manganese oxide nanocomposites as supercapacitor electrode materials and cathode catalysts for improved power production in microbial fuel cells. Powder Technol 18:9053–9060
Wan C, Cheng M, Zhang Q, Ji N (2013) Preparation of MnO2 nanostructures by controlled crystal growth and its pseudocapacitive properties. Powder Technol 235:706–711
Shivajee-Ganesh K, Purusottam-Reddy B, Hussain OM, Mauger A, Julien CM (2016) Influence of Ti and Zr dopants on the electrochemical performance of LiCoO2 film cathodes prepared by rf-magnetron sputtering. Mater Sci Eng B 209:30–36
Acknowledgements
One of the authors, A. Lakshmi Narayana, would like to thank CSIR, New Delhi, for providing fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lakshmi Narayana, A., Dhananjaya, M., Guru Prakash, N. et al. Nanocrystalline Li2TiO3 electrodes for supercapattery application. Ionics 23, 3419–3428 (2017). https://doi.org/10.1007/s11581-017-2147-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-017-2147-1