Abstract
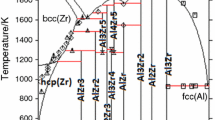
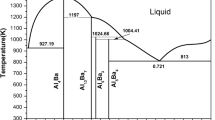
The thermodynamic modelling of the binary Al-Zr system has been carried out using the CALPHAD approach, based on available experimental and theoretical data for phase diagram and thermodynamic properties. A set of self-consistent thermodynamic parameters was established. The liquid phase and the terminal fcc_A1(Al), bcc_A2(Zr), hcp_A3(Zr) solid solutions were treated as disordered solutions, using the Redlich-Kister expressions for the excess Gibbs energy, and the intermetallic phases were considered to be line compounds. A satisfactory agreement was achieved between the experimental data and the calculated results.





Similar content being viewed by others
References
E. Fischer, NUCLEA Thermodynamic Database, Database for Corium Applications (2014) Institut de Radioprotection et Sûreté Nucléaire, St Paul lez Durance
A. Laik, K. Bhanumurthy, and G.B. Kale, Intermetallics in the Zr-Al Diffusion Zone, Intermetallics, 2004, 12(1), p 69
J. Murray, A. Peruzzi, and J.P. Abriata, The Al-Zr (aluminum-zirconium) System, J. Phase Equilib., 1992, 13(3), p 277-291
N. Saunders and V.G. Rivlin, Thermodynamic Characterization of Al-Cr, Al-Zr and Al-Cr-Zr Alloy Systems, Mater. Sci. Technol., 1986, 2, p 521-527
N. Saunders, Calculated Stable and Metastable Phase Equilibria in Al-Li-Zr Alloys, Z. Metallkd., 1989, 80(12), p 894-903
S.V. Meschel and O.J. Kleppa, Standard Enthalpies of Formation of 4d Aluminides by Direct Synthesis Calorimetry, J. Alloys Compd., 1993, 191, p 111-116
R. Klein, I. Jacob, P.A.G. O’Hare, and R.N. Goldberg, Solution-Calorimetric Determination of the Standard Molar Enthalpies of Formation of the Pseudobinary Compounds Zr(AlxFe1−x)2 at the Temperature 298.15 K, J. Chem. Thermodyn., 1994, 26, p 599-608
A. Peruzzi, Reinvestigation of the Zr-rich End of the Zr-Al Equilibrium Phase Diagram, J. Nucl. Mater., 1992, 186, p 89-99
T. Wang, Z. Jin, and J.C. Zhao, Thermodynamic Assessment of the Al-Zr Binary System, J. Phase Equilib., 2001, 22, p 544-551
G. Ghosh and M. Asta, First-Principles Calculation of Structural Energetics of Al-TM (TM = Ti, Zr, Hf) Intermetallics, Acta Mater., 2005, 53, p 3225-3252
H. Zhang and S. Wang, The Structural Stabilities of the Intermetallics and the Solid-State Phase Transformations Induced by Lattice Vibration Effects in the Al-Zr System by First-Principles Calculations, J. Mater. Res., 2010, 25(9), p 1689-1694
M. Mihalkovic, M. Widom and co-workers, Alloy Database, retrieved from http://www.alloy.phys.cmu.edu, online (2011)
C. Colinet, J.-C. Crivello, and J.-C. Tedenac, Structural Stability of Ternary C22-Zr6X2Co (X=Al, Ga, Sn, As, Sb, Bi, Te) and C22-Zr6Sn2T′ (T′=Fe Co, Ni, Cu) Compounds, J. Solid State Chem., 2013, 205, p 217
J.E. Saal, S. Kirklin, M. Aykol, B. Meredig, and C. Wolverton, Materials Design and Discovery with High-Throughput Density Functional Theory : The Open Quantum Materials Database (OQMD), JOM, 2013, 65, p 1501-1509
Y.H. Duan, B. Huang, Y. Sun, M.J. Peng, and S.G. Zhou, Stability, Elastic Properties and Electronic Structures of the Stable Zr-Al Intermetallic Compounds: A First-Principles Investigation, J. Alloys Compd., 2014, 590, p 50-60
H. Okamoto, The Al-Zr System, J. Phase Equilib., 1993, 14(2), p 259-260
H. Okamoto, Al-Zr Aluminum-Zirconium, J. Phase Equilib., 2002, 23(5), p 455
H. Okamoto and T.B. Massalski, Guidelines For Binary Phase Diagram Assessment, J. Phase Equilib., 1993, 14(3), p 316-335
D.J. McPherson and M. Hansen, The System Zr-Al, Trans. ASM, 1954, 46, p 354-374
R.J. Kematick and H.F. Franzen, Thermodynamic Study of the Zirconium-Aluminum System, J. Solid State Chem., 1984, 54, p 226-234
E.M. Schulson, D.H. McColl, and V.C. Ling, Report No. AECL-5176, Atomic Energy of Canada Limited, Chalk River Laboratories, Chalk River, July 1975
S.N. Tiwari and K. Tangri, The Solid Solubility of Aluminum in α-Zirconium, J. Nucl. Mater., 1970, 34, p 92-96
W.L. Fink and L.A. Willey, Equilibrium Relation in Al-Zr Alloys, Met. Technol., 1939, 1, p 69-80
V.M. Glazov, G. Lazarev, and N. Korolkov, The Solubility of Certain Transition Metals in Aluminium, Met. Term. Obrab. Met., 1959, 10, p 48-50
P. Chiotti and P.F. Woerner, Metal Hydride Reactions: I. Reaction of Hydrogen with Solutes in Liquid Metal Solvents, J. Less-Common Met., 1964, 7, p 111-119
M.E. Drits, E.S. Kadaner, and V.K. Kuz’mina, Solubility of Silicon and Zirconium in Aluminium, Izv. Akad. Nauk., 1968, 1, p 102-105
G.M. Kuznetsov, A.D. Barsukov, and M.I. Abas, Solubility of Mn, Cr, Ti, and Zr in Al in the Solid State, Sov. Non Ferrous Met. Res., 1983, 11, p 47-51
A. Janghorban, A. Antoni-Zdziobek, M. Lomello-Tafin, C. Antion, Th Mazingue, and A. Pisch, Phase Equilibria in the Aluminium-Rich Side of the Al-Zr System, J. Therm. Anal. Calorim., 2013, 114(3), p 1015-1020
O. Dezellus, B. Gardiola, and J. Andrieux, On the Solubility of Group IV Elements (Ti, Zr, Hf) in Liquid Aluminum below 800°C, J. Phase Equilib. Diffus., 2014, 35(2), p 120-126
C. Colinet and A. Pasturel, Phase Stability and Electronic Structure in ZrAl3 Compound, J. Alloys Compd., 2001, 319, p 154-161
C. Colinet, Ab-initio Calculation of Enthalpies of Formation of Intermetallic Compounds and Enthalpies of Mixing of Solid Solutions, Intermetallics, 2003, 11, p 1095-1102
G. Kresse and J. Furthmüller, Efficiency of Ab-Initio Total Energy Calculations for Metals and Semiconductors Using a Plane-Wave Basis Set, Comput. Mater. Sci., 1996, 6, p 15-50
G. Kresse and J. Furthmüller, Efficiency of Ab-Initio Total Energy Calculations for Metals and Semiconductors Using a Plane-Wave Basis Set, Phys. Rev. B, 1996, 54, p 11169-11186
D. Vanderbilt, Soft Self-Consistent Pseudopotentials in a Generalized Eigenvalue Formalism, Phys. Rev., 1990, 41, p 7892
J.P. Perdew and Y. Wang, Accurate and Simple Analytic Representation of the Electron-Gas Correlation Energy, Phys. Rev. B, 1992, 45, p 13244
J. Wang, S.-L. Shang, Y. Wang, Z.-G. Mei, Y.-F. Liang, Y. Du, and Z.-K. Liu, First-Principles Calculations of Binary Al Compounds: Enthalpies of Formation and Elastic Properties, CALPHAD, 2011, 35(4), p 562-573
P.E. Blöch, Projector Augmented-Wave Method, Phys. Rev. B, 1994, 50, p 17953-17979
G. Kresse and D. Joubert, From Ultrasoft Pseudopotentials to the Projector Augmented-Wave Method, Phys. Rev. B, 1998, 59, p 1758-1775
J.P. Perdew, S. Burke, and M. Ernzerhof, Generalized Gradient Approximation Made Simple, Phys. Rev. Lett., 1996, 77, p 3865-3868
X.L. Yuan, D.Q. Wei, X.R. Chen, Q.M. Zhang, and Z.Z. Gong, The First-Principles Calculations for the Elastic Properties of Zr2Al under Compression, J. Alloys Compd., 2011, 509, p 769-774
Y. Zhan and M. Pang, Bonding Characteristics and Site Occupancies of Alloying Elements in Zr3Al2 Compound from First Principles, J. Alloys Compd., 2015, 622, p 960-965
X. Tao, J. Zhu, H. Guo, Y. Ouyang, and Y. Du, Phase stability, Thermodynamic and Mechanical Properties of AlZr2, FeZr2 and Al2FeZr6 from First-Principles Calculations, J. Nucl. Mater., 2013, 440, p 6-10
J.-P. Harvey, A.E. Gheribi, and P. Chartrand, Thermodynamic Integration Based on Classical Atomistic Simulations to Determine the Gibbs Energy of Condensed Phases: Calculation of the Aluminum-Zirconium System, Phys. Rev. B, 2012, 86, p 224202
Y.O. Esin, N.N. Serebrennikov, E.D. Pletneva, and V.K. Kapustkin, Temperature Dependence of the Enthalpy and Heat Capacity of Zirconium Aluminides in the Solid and Liquid States, Izv. Vyssh. Ucheb. Zaved. Chern. Metall., 1987, 10, p 1-3, in Russian
Y.O. Esin, N.P. Bobrov, M.S. Petrushevskiii, and P.V. Gel’d, Enthalpy of Formation of Liquid Aluminum Alloys with Titanium and Zirconium, Izv. Akad. Nauk SSSR, Met., 1974, 5, p 104-109, in Russian, Russ. Metall., 1974, 5, p 86-89 (English translation)
G.I. Batalin, V.S. Sudavtsova, and N.N. Maryanchik, Thermodynamic Properties of Liquid Al-Sc, Al-V, and Al-Ti Binary Alloys, Ukr. Khim. Zh., 1985, 51(8), p 817-819
V.S. Sudavtsova, G.I. Batalin, and V.S. Tutevich, Thermodynamic Properties of Molten Binary Alloys in Systems Al-Zr(Nb,Mo), Izv. Akad. Nauk SSSR, Met., 1985, 5, p 185-187, in Russian, Russ. Metall., 1985, 5, p 183-185 (English translation)
V. S. Sudavtsova and N. V. Podoprigora, Thermodynamic Properties of Melts in Al-Ti(Zr,Hf) Binary Systems, Powder Metall. Met. Ceram., 2009, 48(1-2), p 83-87, Translated from Poroshk. Metall., 2009, 48(1-2), p 107-112
V. Witusiewicz, U.K. Stolz, I. Arpshofen, and F. Sommer, Thermodynamics of Liquid Al-Cu-Zr Alloys, Z. Metallkd., 1998, 89, p 704-713
I. Ansara, A. Pasturel, and K.H.L. Buschow, Enthalpy effects in Amorphous Alloys and Intermetallic Compounds in the System Zr-Cu, Phys. Stat. Sol. A, 1982, 69, p 447-453
P.A. Gomozov, Yu.V. Zasypalov and B. M. Mogutnov, Enthalpies of Formation of Intermetallic Compounds with the CsCI Structure (CoTi, CoZr, CoAl, NiTi), Russ. J. Phys. Chem., 1986, 60(8), p 1122-1124, Translated from Zh. Fiz. Khim., 1986, 60, p 1865-1867
K. Nagarajan, R. Babu, and C.K. Mathews, Enthalpy of Formation of UZr2 by Calorimetry, J. Nucl. Mater., 1993, 203, p 221-223
A.A. Turchanin and I.A. Tomilin, Experimental Investigations of the Enthalpies of Formation of Zr-Based Metallic Amorphous Binary and Ternary Alloys, Ber. Bunsenges. Phys. Chem., 1998, 102(9), p 1252-1258
A.A. Zubkov, A.A. Turchanin, and I.A. Tomilin, A High-Temperature Solution Calorimeter for Measuring the Heat of Mixing, Indus. Lab., 1995, 61(9), p 544-547
A. Decreton, P. Benigni, J. Rogez, G. Mikaelian, M. Barrachin, M. Lomello-Tafin, C. Antion, A. Janghorban, and E. Fischer, Contribution to the Description of the Absorber Rod Behavior in Severe Accident Conditions: An Experimental Investigation of the Ag-Zr Phase Diagram, J. Nucl. Mater., 2015, to be published
G.I. Batalin, E.A. Beloborodova, V.V. Nerubaschenko, V.D. Galochka, and L.I. Slyuzko, Thermodynamic Properties of Liquid Solutions in the Aluminum-Zirconium System, Izv. Vyssh. Ucheb. Zaved. Tsvetn. Metall., 1982, 3, p 74-77
A.T. Dinsdale, SGTE Data for Pure Elements, CALPHAD, 1991, 15(4), p 317-425
O. Redlich and A.T. Kister, Algebraic Representation of Thermodynamic Properties and the Classification of Solutions, Ind. Eng. Chem., 1948, 40, p 345-348
H.L. Lukas, ETh Henig, and B. Zimmermann, Optimisation of Phase Diagrams by a Least Squares Method Using Simultaneously Different Types of Data, CALPHAD, 1977, 1(3), p 225-236
M. Potzschke and K. Schubert, Zum Aufbau einiger zu T4-B3 Homologer und Quasihomologer Systeme (To Build some Homology with T4-B3 and Quasi-Homologous Systems), Z. Metallkd., 1962, 53(8), p 548-561
Acknowledgments
This work has been performed in the framework of the development of the NUCLEA thermodynamic database for nuclear materials; it was supported by Universite Grenoble Alpes, CMTC, SIMAP, and IRSN.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fischer, E., Colinet, C. An Updated Thermodynamic Modeling of the Al-Zr System. J. Phase Equilib. Diffus. 36, 404–413 (2015). https://doi.org/10.1007/s11669-015-0398-y
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11669-015-0398-y