Abstract
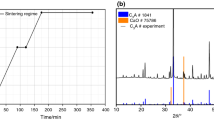
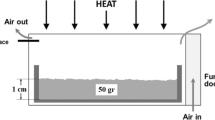
The phase equilibria in the lime rich part of the CaO-Al2O3-B2O3 ternary system were investigated by thermodynamic modeling and key experiments. Three ternary compounds, CaAl2B2O7 (CAB), Ca2Al2B2O8 (C2AB) and Ca2Al2B6O14 (C2AB3), are reported in the literature and their thermodynamic properties were calculated using Density Functional Theory and lattice dynamics theory. Partial isothermal sections of the lime rich part of the CaO-Al2O3-B2O3 ternary system were investigated at 950 and 1020 °C using solid state reactions and x-ray diffraction on 14 selected samples. The observed results confirm the available experimental data from the literature. Based on thermal analysis using differential scanning calorimetry, the Ca3Al2O6-CaB2O4 and CaAl2O4-CaB2O4 T-x sections as well as a tentative partial liquid surface of the lime rich part were constructed.












Similar content being viewed by others
References
E.M. Gartner, Industrially Interesting Approaches to “low-CO2” Cements, Cem. Concr. Res., 2004, 34, p 1489-1498
E.M. Gartner, G. Li, High belite-containing sulfoaluminous clinker, method for the productions and the use thereof for preparing hydraulic binders, Patent WO 2006018569 A3 (2006)
M. Ben Haha, F. Winnefeld, and A. Pisch, Advances in Understanding ye’elimite-rich Cements, Cem. Concr. Res., 2019, 123, p 105778
H.F.W. Taylor, Cement Chemistry, 2nd ed., Thomas Telford Publishing, London, 1997
J.G. Fletcher and F.P. Glasser, Phase Relations in the System CaO-B2O3-SiO2, J. Mater. Sci., 1993, 28, p 2677-2686
P.L. Higby, R.J. Ginther, I.D. Aggarwal, and E.J. Friebele, Glass-Formation and Thermal Properties of Low-Silica Calcium Aluminosilicate Glasses, J. Non-Cryst. Solids, 1990, 126(3), p 209-215
F.T. Wallenberger, R.J. Hicks, and A.T. Bierhals, Design of Environmentally Friendly Fiberglass Compositions: Ternary Eutectic SiO2-Al2O3-CaO Compositions, Structures and Properties, J. Non-Cryst. Solids, 2004, 349, p 377-387
J.F. MacDowell, Aluminoborate Glass-Ceramics with Low Thermal Expansivity, J. Am. Ceram. Soc., 1990, 73(8), p 2287-2292
C. Hirayama, Properties of Aluminoborate Glasses of Group II, Metal Oxides: I, Glass Formation and Thermal Expansion, J. Am. Ceram. Soc., 1961, 44, p 602-606
R. El-Hayek, F. Ferey, P. Florian, A. Pisch, and D.R. Neuville, Structure and Properties of Lime Alumino-Borate Glasses, Chem. Geol., 2017, 461, p 75-81
U.-L. Schäfer and H.-J. Kuzel, Kompatibilitätsbeziehungen und ternäre Verbindungen im System CaO-Al2O3-B2O3, Neues Jahrb. Mineral. Monatsh., 1967, 1967, p 131-136 (in German)
W. Schuckmann, Zur Struktur des Calcium-Aluminium-Borates, CaAl[O7BO3], Neues Jahrb, Miner. Monatsh., 1968, 1968, p 80-86 (in German)
K.S. Chang and D.A. Keszler, CaAl2(BO3)2O: Crystal Structure, Mater. Res. Bull., 1998, 33, p 299-304
E. Iwase and N. Saito, Johachidolite: A New Mineral, a Hydrous Fluoborate of Sodium, Calcium and Aluminum, Sci. Pap. Inst. Phys. Chem. Res. (Tokyo), 1942, 39, p 300-304, in Japanese
P.B. Moore and T. Araki, Johachidolite, CaAl[B3O7], A Borate with Very Dense Atomic Structure, Nat. Phys. Sci., 1972, 240, p 63-65
M. Kadiyski, T. Armbruster, D. Günther, E. Reusser, and A. Peretti, Johachidolite, CaAl[B3O7], A Mineralogical and Structural Peculiarity, Eur. J. Miner., 2008, 20, p 965-973
N.I. Leonyuk, Structural Aspects in Crystal Growth of Anhydrous Borates, J. Cryst. Growth, 1997, 174, p 301-307
R.R. Harding, J.G. Francis, C.J.E. Oldershaw, and A.H. Rankin, Johachidolite—A New Gem, J. Gemm., 1999, 26(5), p 324-329
C.W. Bale, E. Bélisle, P. Chartrand, S.A. Decterov, G. Eriksson, A.E. Gheribi, K. Hack, I.-H. Jung, Y.-B. Kang, J. Melançon, A.D. Pelton, S. Petersen, C. Robelin, J. Sangster, P. Spencer, and M.-A. Van Ende, FactSage Thermochemical Software and Databases 2010–2016, Calphad, 2016, 54, p 35-53
E.T. Carlson, The System: CaO-B2O3, J. Res. Nat. Bur. Stand., 1932, 9, p 825
S.A. Decterov, V. Swamy, and I.-H. Jung, Thermodynamic Modeling of the B2O3-SiO2 and B2O3-Al2O3 System, Int. J. Mater. Res., 2007, 98, p 988-994
D. Mazza, M. Vallino, and G. Busca, Mullite-Type Structures in the Systems Al2O3–Me2O (Me = Na, K) and Al2O3–B2O3, J. Am. Ceram. Soc., 1992, 75(7), p 1929-1934
R.W. Nurse, J.H. Welch, and A.J. Majumdar, The CaO–Al2O3 System in a Moisture-Free Atmosphere, Trans. Br. Ceram. Soc., 1965, 64, p 409-418
D.A. Jerebtsov and G.G. Mikhailov, Phase Diagram of CaO–Al2O3 System, Ceram. Int., 2001, 27, p 25-28
R.W. Nurse, J.H. Welch, and A.J. Majumdar, The 12CaO·7Al2O3 Phase in the CaO–Al2O3 System, Trans. Br. Ceram. Soc., 1965, 64, p 323-332
A.K. Chatterjee and G.I. Zhmoidin, The Phase Equilibrium Diagram of the System CaO-Al2O3-CaF2, J. Mater. Sci., 1972, 7, p 93-97
P. Hohenberg and W. Kohn, Inhomogeneous Electron Gas, Phys. Rev., 1964, 136, p B864-B871
W. Kohn and L.J. Sham, Self-consistent Equations Including Exchange and Correlation Effects, Phys. Rev., 1965, 140, p A1133-A1138
G. Kresse and J. Furthmüller, Efficient Iterative Schemes for Ab Initio Total-Energy Calculations Using a Plane-Wave Basis Set, Phys. Rev. B, 1996, 54, p 11169-11186
G. Kresse and D. Joubert, From Ultrasoft Pseudopotentials to the Projector Augmented-Wave Method, Phys. Rev. B, 1999, 59, p 1758-1775
J. Sun, A. Ruzsinszky, and J.P. Perdew, Strongly Constrained and Appropriately Normed Semilocal Density Functional, Phys. Rev. Lett., 2015, 115, p 036402
D.A. Kitchaev, H. Peng, Y. Liu, J. Sun, J.P. Perdew, and G. Ceder, Energetics of MnO2 Polymorphs in Density Functional Theory, Phys. Rev., 2016, B93, p 045132
H.J. Monkhorst and J.D. Pack, Special Points for Brillouin-Zone Integrations, Phys. Rev., 1976, B13, p 5188-5192
P.E. Blöchl, O. Jepsen, and O.K. Andersen, Improved Tetrahedron Method for Brillouin-Zone Integrations, Phys. Rev., 1994, B49, p 16223-16233
A. Togo and I. Tanaka, First Principles Phonon Calculations in Materials Science, Scripta Mater., 2015, 108, p 1-5
Acknowledgment
The authors acknowledge fruitful discussions within the French research consortium on high temperature thermodynamics GDR 3584 “TherMatHT” (www.thermatht.fr). CIMENT/GRICAD in the frame of the “atosimul” project is acknowledged for computational resources.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This invited article is part of a special tribute issue of the Journal of Phase Equilibria and Diffusion dedicated to the memory of Günter Effenberg. The special issue was organized by Andrew Watson, Coventry University, Coventry, United Kingdom; Svitlana Iljenko, MSI, Materials Science International Services GmbH, Stuttgart, Germany; and Rainer Schmid-Fetzer, Clausthal University of Technology, Clausthal-Zellerfield, Germany.
Rights and permissions
About this article
Cite this article
Ferey, F., Briaud, V., Violet, P. et al. Experimental and Theoretical Contribution to the Phase Equilibria in the Ternary CaO-Al2O3-B2O3 System. J. Phase Equilib. Diffus. 41, 443–456 (2020). https://doi.org/10.1007/s11669-020-00803-7
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11669-020-00803-7