Abstract
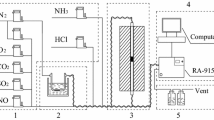
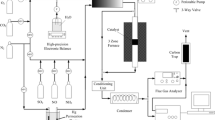
The performance of V2O5/TiO2-based commercial SCR catalyst for the oxidation of gaseous elemental mercury (Hg0) with respect to reaction conditions was examined to understand the mechanism of Hg0 oxidation on SCR catalyst. It was observed that a much larger amount of Hg0 adsorbed on the catalyst surface under oxidation condition than under SCR condition. The activity of commercial SCR catalyst for Hg0 oxidation was negligible in the absence of HCl, regardless of reaction conditions. The presence of HCl in the reactant gases greatly increased the activity of SCR catalyst for the oxidation of Hg0 to oxidized mercury (Hg2+) such as HgCl2 under oxidation condition. However, the effect of HCl on the oxidation of Hg0 was much less under SCR condition than oxidation condition. The activity for Hg0 oxidation increased with the decrease of NH3/NO ratio under SCR condition. This might be attributed to the strong adsorption of NH3 prohibiting the adsorption of HCl which was vital species promoting the oxidation of Hg0 on the catalyst surface under SCR condition.
Similar content being viewed by others
References
S. E. Lindberg and W. J. Stratton, Environ. Sci. Technol., 32, 49 (1998).
C. C. Travis and B. P. Blaylock, Toxicol. Environ. Chem., 49, 203 (1995).
U. S. Government Printing Office, Mercury study report to congress, Washington, DC (1997).
U. S. Government Printing Office, A study of hazardous air pollutant from electric utility steam generating units: Final report to congress, Washington, DC (1998).
U. S. Environmental Protection Agency, U. S. EPA clean air mercury rule, Washington, DC (2005).
J. C. S. Chang and S. B. Ghorishi, Environ. Sci. Technol., 37, 5763 (2003).
P. S. Nolan, K. E. Redinger, G. T. Amrhein and G. A. Kudlac, Fuel Process Technol., 85, 587 (2004).
R. D. Vidic and D. P. Siler, Carbon, 39, 3 (2001).
S. V. Krishnan, B. K. Gullett and W. Jorewlczt, Environ. Sci. Technol., 28, 1506 (1994).
R. D. Vidic and J. B. McLaughlin, J. Air Waste Manage. Assoc., 46, 241 (1996).
W. J. O’Dowd, R. A. Hargis, E. J. Granite and H. W. Pennline, Fuel Process Technol., 85, 533 (2004).
E. Pitoniak, C. Y. Wu, D. W. Mazyck, K. W. Powers and W. Sigmund, Environ. Sci. Technol., 39, 1269 (2005).
J. W. Portzer, J. R. Albritton, C. C. Allen and R. P. Gupta, Fuel Process Technol., 85, 621 (2004).
E. J. Granite, H. W. Pennline and R. A. Hargis, Ind. Eng. Chem. Res., 39, 1020 (2000).
T. Garey, in Proceedings of the Air and Waste Management Association’s 92 nd Annual Meeting, June, Pittsburgh PA (1999).
S. Niksa and N. Fujiwara, J. Air Waste Manage. Assoc., 55, 1866 (2005).
S. Straube, T. Hahn and H. Koeser, Appl. Catal. B: Environ., 79, 286 (2008).
C. Lee, R. Srivastava, S. Ghorishi, T. Hastings and F. Stevens, J. Air Waste Manage. Assoc., 54, 1560 (2004).
G. Dunham, R. DeWall and C. Senior, Fuel Process Technol., 82, 197 (2003).
E. Olsen, S. Miller, R. Sharma, G. Dunham and S. Benson, J. Hazard. Mater., 74, 61 (2000).
S. Kellie, Y. Cao, Y. Duan, L. Li, P. Chu, A. Mehta, R. Carty, J. Riley and W. Pan, Energy Fuels, 19, 800 (2005).
S. Ghorishi, C. Lee, W. Jozewicz and J. Kilgroe, Environ. Eng. Sci., 22, 221 (2005).
Y. Zhao, M. Mann, J. Pavlish, B. Mibeck, G. Dunham and E. Olson, Environ. Sci. Technol., 40, 1603 (2006).
J. Pavlish, E. Sondreal, M. Mann, E. Olson, K. Galbreath, D. Laudal and S. Benson, Fuel Process Technol., 82, 89 (2003).
S. W. Ham and I. S. Nam, Catalysis Vol. 16, Ed. J. J. Spivey, The Royal Society of Chemistry, Cambridge, 236 (2002).
S. C. Choo, I. S. Nam, S. W. Ham and J. B. Lee, Korean J. Chem. Eng., 20(2), 273 (2003).
S. W. Ham, I. S. Nam and Y. G. Kim, Korean J. Chem. Eng., 17(3), 318 (2000).
A. Miyamoto, Y. Yamazaki, T. Hattori, M. Inomata and Y. Murakami, J. Catal., 74, 144 (1982).
S. C. Wu and K. Nobe, Ind. Eng. Chem. Prod. Res. Dev., 16, 136 (1977).
A. A. Presto and E. J. Granite, Environ. Sci. Technol., 40, 5601 (2006).
A. Miyamoto, M. Inomata, Y. Yamazaki and Y. Murakami, J. Catal., 57, 526 (1979).
M. Inomata, A. Miyamoto and Y. Murakami, J. Catal., 62, 140 (1980).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hong, HJ., Ham, SW., Kim, M.H. et al. Characteristics of commercial selective catalytic reduction catalyst for the oxidation of gaseous elemental mercury with respect to reaction conditions. Korean J. Chem. Eng. 27, 1117–1122 (2010). https://doi.org/10.1007/s11814-010-0175-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-010-0175-x