Abstract
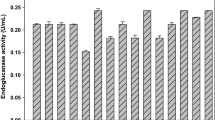
The activity of β-glucosidase (βG), total cellulase (FPase) and endoglucanase (CMCase), produced by Aspergillus japonicus URM5620, was studied on solid-state fermentation using castor bean meal as substrate. The effect of the substrate amount, initial moisture, pH, and temperature on cellulase production was studied using a full factorial design (24). The maximum βG, FPase, and CMCase activity was 88.3, 953.4, and 191.6 U/g dry substrate, respectively. The best enzyme activities for all three enzymes were obtained at the same conditions with 5.0 g of substrate, initial moisture 15% at 25 °C and pH 6.0 with 120 h of fermentation. The optimum activity for FPase and CMCase was found at pH 3.0 at an optimum temperature of 50 °C for FPase and of 55 °C for CMCase. The cellulases were stable in the range of pH 3.0–10.0 at 50 °C temperature. The enzyme production optimization demonstrated clearly the impact of the process parameters on the yield of the cellulolytic enzymes.



Similar content being viewed by others
References
Godoy, M. G., Gutarra, M. L. E., Maciel, F. M., Felix, S. P., Bevilaqua, J. V., Machado, O. L. T., et al. (2009). Use of a low-cost methodology for biodetoxification of castor bean waste and lipase production. Enzyme and microbial technology, 44, 317–322.
Audi, J., Belson, M., Patel, M., Shier, J., & Osterloh, J. (2005). Ricin poisoning – A comprehensive review. J. Am. Medical. Assoc., 294, 2342–2351.
Thorpe, S. C., Kemeny, D. M., Panzani, R. C., Mcgurl, B., & Lord, M. (1988). Allergy to castor bean. II. Identification of the major allergens in castor bean-seeds. The Journal of Allergy and Clinical Immunology, 21, 67–72.
Brand, D., Pandey, A., Roussos, S., & Soccol, C. R. (2000). Biological detoxification of coffee husk by filamentous fungi using a solid state fermentation system. Enzyme and Microbial Technology, 26, 127–133.
Hölker, U., Höfer, M., & Lenz, J. (2004). Biotechnological advantages of laboratory-scale solid state fermentation with fungi. Applied Microbiology and Biotechnology, 64, 175–186.
Gao, J., Weng, H., Zhu, D., Yuan, M., Guan, F., & Xi, Y. (2008). Production and characterization of cellulolytic enzymes from the thermoacidophilic fungal Aspergillus terreus M11 under solid-state cultivation of corn stover. Bioresource Technol., 99, 7623–7629.
Bhat, M. K., & Bhat, S. (1997). Cellulose degrading enzymes and their potential industrial application. Biotechnology Advances, 15(3), 583–620.
Harhangi, H. R., Steenbakkers, P. J. M., Akhmanova, A., Jetten, M. S. M., Drift, C. V. D., Camp, O. D., et al. (2002). A highly expressed family 1 β-glucosidase with transglycosylation capacity from the anaerobic fungus Piromyces sp. E2. Biochimica et Biophy. Acta., 1574(3), 293–303.
Bhat, M. K. (2000). Cellulases and related enzymes in biotechnology. Biotechnology Advances, 18, 355–383.
Polizeli, M. L. T. M., Rizzatti, A. C. S., Monti, R., Terenzi, H. F., Jorge, J. A., & Amorim, D. S. (2005). Xylanases from fungi: properties and industrial applications. Applied Microbiology and Biotechnology, 67, 577–591.
Klich, M. A. (2002). Identification of common Aspergillus species (1st ed.). Utrecht: Centraalbureau voor Schimmelcultures.
Spier, M. R., Greiner, R., Rodriguez-León, J. A., Woiciechowski, A. L., Pandey, A., Soccol, V. T., et al. (2008). Phytase production using citric pulp in SSF. Food Technol. Biotechnol., 46, 178–182.
Zenebon, O., & Pascuet, N. S. (2005). Métodos físico-químicos para análise de alimentos (4th ed.). São Paulo: Instituto Adolfo Lutz.
Bruns, R. E., Scarminio, I. S., & Neto, B. B. (2006). Statistical Design-Chemometrics (1st ed.). Elsevier: Amsterdam.
Statsoft (2008), Inc. STATISTICA (data analysis software systems) version 8.0.
Bradford, M. M. (1976). A rapid and sensitive method for the quantification of protein utilizing the principle of protein–dye binding. Analytical Biochemistry, 72, 248–254.
Ghose, T. K. (1987). Measurement of cellulase activities. Pure and Applied Chemistry, 59, 257–268.
Deshpande, V., and Eriksson, K.E. 1,4-ß-glucosidases of Sporotrichum pulverulentum. In: Wood, W.A.; Kellogg, S.T. (Ed.). (1988), Methods in Enzymology. San Diego: Academic Press, 160: 415–424.
Miller, G. L. (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugars. Analytical Chemistry, 31, 426–428.
Herculano, P. N., Lima, D. M. M., Fernandes, M. J. S., Neves, R. P., Souza-Motta, C. M., & Porto, A. L. F. (2011). Cellulases from two Penicillium sp. strains isolated from subtropical forest soil: production and characterization. Current Microbiology, 62, 1416–1422.
Laufenberg, G., Rosato, P., & Kunz, B. (2004). Adding value to vege table waste: oil press cakes as substrates for microbial decalactone production. Eur. J. Lipid Sci. Technol., 106, 207–217.
Hui, L., Wan, C., Hai-Tao, D., Xue-Jiao, C., Qi-Fa, Z., & Yu-Hua, Z. (2010). Direct microbial conversion of wheat straw into lipid by a cellulolytic fungus of Aspergillus oryzae A-4 in solid-state fermentation. Bioresource Technol., 101, 7556–7562.
Nazir, A. S., Saini, R. H. S., Kaur, A., & Chadha, B. S. (2010). Profiling Differential Expression of Cellulases and Metabolite Footprints in Aspergillus terreus. Applied Biochemistry and Biotechnology, 162, 538–547.
Xin, F., & Geng, A. (2010). Horticultural Waste as the Substrate for Cellulase and Hemicellulase Production by Trichoderma reesei Under Solid-State Fermentation. Applied Biochemistry and Biotechnology, 162, 295–306.
Gottschalk, L. M. F., Oliveira, R. A., & Bon, E. P. S. (2010). Cellulases, xylanases, β-glucosidase and ferulic acid esterase produced by Trichoderma and Aspergillus act synergistically in the hydrolysis of sugarcane bagasse. Biochemical Engineering Journal, 51, 72–78.
Dogaris, I., Vakontios, G., Kalogeris, E., Mamma, D., & Kekos, D. (2009). Induction of cellulases and hemicellulases from Neurospora crassa under solid-state cultivation for bioconversion of sorghum bagasse into ethanol. Industrial Crops and Products, 29, 404–411.
Mamma, D., Kourtoglou, E., & Christakopoulos, P. (2008). Fungal multienzyme production on industrial by-products of the citrus-processing industry. Bioresource Technology, 99, 2373–2383.
Botella, C., Diaz, A., Ory, I., Webb, C., & Blandino, A. (2007). Xylanase and pectinase production by Aspergillus awamori on grape pomace in solid state fermentation. Process Biochemistry, 42, 98–101.
Christen, P., Bramorski, A., Revaha, S., & Soccol, C. R. (2000). Characterization of volatile compounds produced by Rhizopus strains grown on agro-industrial solid wastes. Bioresource Technology, 71, 211–215.
Alam, M. Z., Muyibi, S. A., & Wahid, R. (2008). Statistical optimization of process conditions for cellulase production by liquid state bioconversion of domestic wastewater sludge. Bioresource Technology, 99, 4709–4716.
Prasetyo, J., Sumita, S., Okuda, N., Park, E. Y., & Park, E. Y. (2010). Response of Cellulase Activity in pH-Controlled Cultures of the Filamentous Fungus Acremonium cellulolyticus. Applied Biochemistry and Biotechnology, 162, 52–61.
Dutta, T., Sahoo, R., Sengupta, R., Ray, S. S., Bhattacharjee, A., & Ghosh, S. (2008). Novel cellulases from an extremophilic filamentous fungi Penicillium citrinum: production and characterization. J. Ind. Microb. Biotechnol., 35, 275–282.
Gowthaman, M. K., Krishna, C., & Moo-Young, M. (2001). Fungal solid state fermentation – an revision. Appl. Mycol. Biotechnol., 1, 305–352.
Krishna, C. (2005). Solid-State Fermentation Systems – An Overview. Critical Rev. Biotechnol., 25, 1–30.
Picart, P., Diaz, P., & Pastor, F. I. J. (2007). Cellulases from two Penicillium sp. strains isolated from subtropical forest soil: production and characterization. Letters in Applied Microbiology, 45, 108–113.
Sreenath, H. K., Yang, V. W., Burdsall, H. H., Jeffries, T. W. (1996). Toner removal by alkaline-active cellulases from desert Basidiomycetes. In: Jeffries T.W., Viikari, L. (Eds.), The enzymes for pulp and paper processing. Washington: American Chemical Society, 267–279.
Acknowledgments
The authors acknowledge FINEP ref. 2083/07—RENNEBRA/CNPq process no. 552410/2005-5, RENORBIO/MCT/CNPq process no. 554740/2006-0, and CAPES for providing financial support to this research work. The authors also gratefully acknowledge the Nogueira, E.B.S. and the Micoteca URM, of the Mycology Department at UFPE, Brazil.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Herculano, P.N., Porto, T.S., Moreira, K.A. et al. Cellulase Production by Aspergillus japonicus URM5620 Using Waste from Castor Bean (Ricinus communis L.) Under Solid-State Fermentation. Appl Biochem Biotechnol 165, 1057–1067 (2011). https://doi.org/10.1007/s12010-011-9321-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-011-9321-0