Abstract
In recent times, East Kolkata Wetlands (EKW), a designated Ramsar site in the eastern part of megacity Kolkata, has been threatened by toxic heavy metal (HM) pollution. Besides being a natural wetland supporting biodiversity, EKW serves as a significant food basket for the city. For assessing the magnitude of HM pollution in this wetland, the three most cultivated food crops of EKW, namely Lagenaria siceraria (bottle gourd), Abelmoschus esculentus (ladies’ fingers), and Zea mays (maize), as well as the ambient soil samples, were collected during premonsoon, monsoon, and postmonsoon for 2 consecutive years (2016 and 2017). Predominant HMs like cadmium (Cd), chromium (Cr), mercury (Hg), and lead (Pb) were analyzed in the roots and edible parts of these plants, as well as in the ambient soil to evaluate the bioaccumulation factor (BF) and translocation factor (TF) of each HM in the three vegetables. It was observed that the HM content in the food crop species followed the order Z. mays > L. siceraria > A. esculentus. HMs accumulated in all three vegetables as per the order Pb > Cd > Cr > Hg. Monsoon seems to be threatening in terms of bioaccumulation and translocation of HMs as both BF and TF were highest in this season irrespective of the plant species. Hence it demands critical monitoring of HM pollution levels in this wetland and subsequent ecorestoration through distinctive plant growth-promoting rhizobacteria (PGPR)-assisted co-cultivation of these food crops with low-metal-accumulating, deep-rooted, high-biomass-yielding, and bioenergy-producing perennial grass species for minimizing HM intake.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the recent past, population explosion has augmented urbanization, industrialization, and intensive agricultural activities to meet the increasing demand for food, eventually leading to greater anthropogenic stress on the environment. A key challenge faced by modern society today is environmental pollution, of which one of the severe threats is heavy metal (HM) toxicity. Life processes on earth, such as the growth of plants and animals, depend on some metals in trace amounts, which are essential and considered micronutrients. However, certain forms of some metals can be toxic, even in relatively trace concentrations, and therefore, pose risks to the overall health and well-being of plants, animals, and humans [1, 2]. The HMs, viz., arsenic (As), cadmium (Cd), chromium (Cr), copper (Cu), mercury (Hg), and lead (Pb), have no known physiological functions, but depending on their amount in the human body tissues, there is high probability of adverse impact on the overall health [3,4,5,6]. In addition to this, these HMs also negatively affect the overall growth and development of cultivable plants, including food crops (e.g., biomass, photosynthetic rate, chlorophyll a and b content) [7]. Unlike organic contaminants, HMs do not have an environmental half-life but persist indefinitely in the environment. They can only be transformed from more toxic to less or non-toxic forms by the action of microorganisms, including bacteria and fungi [8]. HMs entering food via anthropogenic or natural contamination of air, water, or soil are carried in the food chain by plants and animals because they tend to bioaccumulate in the living tissues [9].
The problem of HM pollution is more pronounced in developing countries than in developed nations. India, the second most populous country globally, with enormous and rich biodiversity, is no exception. Environmental HM toxicity affects biodiversity, and the maritime state of West Bengal in the eastern part of India offers incredible biodiversity owing to the presence of two world-renowned biodiversity hotspots, namely the Indian Sundarbans and the East Kolkata Wetlands (EKW). The former is a UNESCO World Heritage Site, and the latter is a designated Ramsar site. EKW provides a unique example of the participation of the local people in the process of its restoration and upgradation and is a world famous model of a wetland, which serves multiple purposes, such as resource recovery, flood control, detoxification of the environment, carbon sequestration, habitat for a variety of flora and fauna, and livelihood support for the local farmers [10, 11]. It has saved the city from colossal construction and maintenance costs associated with wastewater treatment plants. Discharge of wastewater in the EKW sites often contaminates the associated edible flora and fauna with HMs, most of which are often toxic.
The present study was carried out to assess the magnitude of HM pollution in EKW, a complex of natural and artificial wetlands lying east of the metropolitan city of Kolkata, West Bengal, in India. The city of Kolkata, with a total area of 1886.67 km2 (out of which 206.08 km2 is within the Kolkata Municipal Corporation (KMC) area) (https://en.wikipedia.org/wiki/Kolkata), sustains ~ 15.13 billion people, as reviewed in 2022, due to which the population density is enormously large (https://worldpopulationreview.com/world-cities/kolkata-population). The wetlands cover 125 km2 and include marshes and meadows as well as sewage farms and settling ponds. The wetlands are used to treat or absorb the sewage wastes of Kolkata, and the various nutrients contained in the wastewater simultaneously sustain pisciculture and agriculture in adjoining fish farms and farmlands, respectively. In EKW, the total fresh vegetables harvested daily are about 150 tons and fish cultivated per year is 10,500 tons [12]. The EKW was designated a “wetland of international importance” under the Ramsar Convention on 19 August 2002. This Ramsar site consists of 264 fish ponds managing wastewater aquaculture, horticultural plots, agricultural land, and residential area [13]. It is a unique resource recovery ecosystem where pond-effluent-based rice cultivation, garbage-based vegetable farming, sewage-fed aquaculture, and agriculture are practiced [14]. The presence of and seasonal variations in toxic HMs like Cd, Cr, Pb, and Hg have been reported by Dutta et al. [15] in the tissues of three commonly cultivated edible carp variety of fishes, namely Rohu (Labeo rohita), Catla (Catla catla), and Nile tilapia (Oreochromis niloticus), cultivated in EKW ponds (locally known as “bheries”).
Around 100 different plant species have been recorded in and around the EKW [16]. A variety of vegetables are farmed here, including bottle gourd, ladies’ fingers, cauliflower, corn, eggplant, pumpkin, and sacred basil [17]. In addition, vast tracts of land are dedicated to paddy cultivation [17]. The three commonly used vegetables, namely Lagenaria siceraria, Abelmoschus esculentus, and Zea mays, along with their ambient soil, were collected from the Dhapa waste dumping site in EKW through three seasons (premonsoon, monsoon, and postmonsoon) in 2 consecutive years 2016 and 2017, and the selected HM contents of Cd, Cr, Pb, and Hg were analyzed in the edible parts of these plants along with their root systems.
L. siceraria (bottle gourd), possibly the first domesticated vegetable species, is used as food and medicine and for other uses like making utensils and musical instruments [18]. A. esculentus (ladies’ fingers) is also an economically important vegetable crop, quite popular in India because of the ease of cultivation, dependable yield, and adaptability to varying moisture conditions [19, 20]. Z. mays (corn) is a tall monoecious annual grass having high calorific value and nutritional content. It is a staple diet of many inhabitants worldwide [21, 22].
Vegetables are essential dietary constituents and contain water, carbohydrates, proteins, vitamins, minerals, phenolic compounds, volatiles, and antioxidants [23]. Vegetables are connected to improved human health in terms of better vision, reduced risk of heart-related ailments, controlling blood sugar, and gastrointestinal health. Some vegetables have potent antioxidants, which protect the body from reactive oxygen species (ROS). Each vegetable has a unique combination of phytochemicals, and hence only a variety of vegetables can ensure better health benefits.
Like fishes, intake of contaminated vegetables and fruits is a risky affair for the health of individuals [24, 25]. Plants can uptake and bioaccumulate HMs from the contaminated ambient soil through their roots as well as absorb the airborne metal deposits on their aboveground parts [26, 27]. Another cause of contamination can be irrigation with contaminated water, such as wastewater from sewage canals [28, 29]. A fundamental matter of grave concern is that these vegetables can be a prolific accumulator of HMs, especially from soil [30], providing an easy entry of these toxic HMs into the food chain. It is well established that HMs exist in the soil both as soluble forms and in combined forms, as salt complexes. Indeed, only soluble, exchangeable, and chelated metal species in the soils are mobile, and therefore, they are made available to the plants [31, 32]. However, HM bioaccumulation in plants depends on vegetable species, growth stages, soil composition, geographic and atmospheric conditions, and metal types and their bioavailability [32,33,34]. Unknowingly, people consume vegetables with high HM content, which, when exceeding the permissible limit, impacts their health leading to various medical disorders [25]. There exists a correlation between the concentrations of HMs in the vegetables with the corresponding soil HM content [35].
The present study aims to investigate whether L. siceraria, A. esculentus, and Z. mays could accumulate biologically available forms of selected HM species (like Cd, Cr, Pb, and Hg) from ambient soil in their roots and edible parts, which might be a direct pathway for HM incorporation into the human food chain, causing many adverse health effects. A few mitigating measures to lessen such toxic metal accumulation within dietary vegetable species through co-cultivation with deep-rooted, high-biomass-producing, and bioenergy-generating perennial grass species [36], along with targeted inoculation of vegetable roots/rhizosphere soils with multi-metallotolerant and HM-accumulating plant growth-promoting rhizobacteria (PGPR) [37], have been proposed.
Materials and Methods
The present research work was carried out through three seasons, namely premonsoon, monsoon, and postmonsoon, for 2 consecutive years (2016 and 2017). The entire research methodology is divided into four sections, as highlighted.
-
i)
Selection of the study site
-
ii)
Sampling and processing of selected vegetable species and ambient soil
-
iii)
Spectrophotometric analysis of selected HMs
-
iv)
Determining the potential for HM bioaccumulation and translocation
Study Site Selection
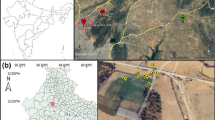
Dhapa (22° 32′ 17.82″N; 88° 25′ 59.19″E), a municipal solid waste (MSW) dumping station in EKW (22° 33′ 13.40″N; 88° 26′ 41.34″E), was selected as the sampling site, which is located in the eastern part of Kolkata, West Bengal. The city of Kolkata is the capital of West Bengal, a maritime state in the northeast part of the Indian subcontinent (Fig. 1).
Map showing the location of the study site, Dhapa (indicated with a red balloon) in East Kolkata Wetlands (22° 32′ 17.82″N; 88° 25′ 59.19″E), situated in the eastern fringe of the metropolitan city of Kolkata, West Bengal, India (source: Google Earth; earth.google.com/web/)
Sample Collection and Processing
Three common vegetables were chosen for the present study, namely L. siceraria, A. esculentus, and Z. mays, as represented in Fig. 2. The edible parts of L. siceraria, A. esculentus, and Z. mays, their root system, and the ambient soil samples were obtained by sampling from the EKW during the premonsoon, monsoon, and postmonsoon seasons in the years 2016 and 2017. All the selected vegetable samples were washed with deionized water and oven-dried at 60 °C overnight.
Heavy Metal Analysis Using Atomic Absorption Spectrometry
The vegetable samples, including the roots and edible parts, were powdered and weighed to determine the HM concentrations using atomic absorption spectrometry (AAS). Each dried sample (1 g on a dry weight basis) was digested with a mixture of nitric acid (HNO3) and hydrogen peroxide (H2O2), followed by the addition of hydrochloric acid (HCl) [38]. The digested samples were analyzed for Cd, Cr, Pb, and Hg against a standard concentration of each metal on a PerkinElmer Atomic Absorption Spectrophotometer (Model 3030) equipped with an HGA-500 graphite furnace atomizer and a deuterium background corrector. The blank correction was done to bring accuracy to the results. The methodology of HM assessment in the selected vegetable samples is illustrated with the help of a flow diagram, as detailed in Fig. 3.
A quantity of approximately 0.5 g of ambient soil samples was digested using a weak acid treatment (i.e., 0.5 N HCl) (37%, Sigma-Aldrich), following the procedure of Malo [39]. Biologically available HM concentrations (Cd, Cr, Pb, and Hg) in the soil samples were analyzed against a standard concentration of each metal in the same instrument as above. For checking the instrument’s analytical precision, the samples (around 20% of the total numbers) were chosen randomly and measured in triplicates against internationally used soil Standard Reference Materials (SRMs): NIST SRM 2709, 2710, and 2711. Average recoveries (n = 5) ranged from 85 to 99% for the analyzed HMs.
Heavy Metal Bioaccumulation and Translocation Potential
Bioaccumulation Factor Estimation
The bioaccumulation factor (BF) is the ratio of HMs within the plant body parts relative to ambient soil. BF of HMs within the roots and edible parts of the selected vegetables from the soil was determined mathematically from the Eqs. (1–2) as given by Mukherjee et al. [37] with some modifications given below:
For roots,
For edible parts,
where Cr and Cep represent HM concentrations in the roots and edible parts of the food crop species under study, respectively, and Cas represents HM concentrations in the ambient soil of the plant species under investigation.
Translocation Factor Estimation
With some modification, the translocation of HMs from the roots to the edible parts of the selected plant species was calculated using Eq. (3), as given by Mukherjee et al. [37]. The translocation factor (TF) is the ratio of HMs in the plant’s aboveground biomass (edible parts in this case) relative to roots.
where Cep represents HM concentrations in the edible parts of the food crop species under study, and Cr represents HM concentrations in the roots of the plant species collected from the study area.
Statistical Analyses
A two-way analysis of variance (ANOVA) was performed to determine if the HM concentration varied significantly between species and seasons; a p value of < 0.01 was considered significant. All analyses were done in triplicates, and the mean values are represented here. The correlation coefficient was calculated to determine the interrelationship between HM concentration in the three selected vegetable species’ ambient soil, roots, and edible parts. Microsoft Excel was used to prepare the graphs and SPSS 21.0 (for Windows) was used for the statistical analyses.
Results
Biologically Available Heavy Metals in Ambient Soil
The trend of the selected HM concentrations in the ambient soil samples was Cr (298.76–443.85 ppm) > Pb (12.02–45.22 ppm) > Cd (1.69–7.68 ppm) > Hg (0.98–1.81 ppm) for 2016 and 2017 with the highest value in premonsoon, followed by postmonsoon and monsoon, respectively (Fig. 4).
Bioaccumulated Heavy Metals in Belowground Biomass (Roots) and Aboveground Biomass (Edible Parts)
Roots
The trend of HM accumulation was the same in the roots of the three selected vegetable species, which varied as per the order Pb > Cd > Cr > Hg in both the years. For all HMs, the highest values were observed in the three selected vegetable roots during monsoon in both years except for Cr and Hg, where the highest values were observed during 2016 postmonsoon in the case of L. siceraria. The HMs (Pb, Cd, Cr, and Hg) in the L. siceraria roots ranged from 26.67 to 39.75 ppm, 9.89 to 17.58 ppm, 7.08 to 12.83 ppm, and 4.89 to 6.26 ppm, respectively, during the entire study period in the chosen site. The range of Pb, Cd, Cr, and Hg in the roots of A. esculentus was 14.66–30.17 ppm, 5.32–11.24 ppm, 2.76–7.16 ppm, and 0.59–2.45 ppm, respectively, while for Z. mays, the range was 33.67–42.39 ppm, 13.97–20.57 ppm, 11.98–19.35 ppm, and 1.37–4.99 ppm for Pb, Cd, Cr, and Hg, respectively. To sum up, the overall seasonal trend of HM bioaccumulation in the roots of the three selected food crop species is monsoon > postmonsoon > premonsoon, with exceptions mentioned above.
Edible Parts
The data revealed that out of the four HMs studied in the three selected vegetables, the concentration of the HMs in the edible parts followed the trend Pb > Cd > Cr > Hg, and the highest values were observed during monsoon, followed by postmonsoon and premonsoon, respectively, for both the study years 2016 and 2017 (Figs. 5, 6, 7). Among the selected vegetables, the HM content was found to be highest in Z. mays and lowest in A. esculentus.
The HMs (Pb, Cd, Cr, and Hg) in edible parts of L. siceraria ranged from 23.59 to 35.45 ppm, 5.88 to 13.98 ppm, 2.04 to 7.63 ppm, and 0.88 to 2.04 ppm, respectively, during the entire study period in the chosen site. The range of Pb, Cd, Cr, and Hg in the edible parts of A. esculentus was 13.51–29.67 ppm, 3.42–9.64 ppm, 1.86–5.56 ppm, and 0.00–1.04 ppm, respectively, while for Z. mays, the range was 31.78–42.33 ppm, 11.93–18.43 ppm, 9.83–17.21 ppm, and 0.45–3.52 ppm for Pb, Cd, Cr, and Hg, respectively.
Bioaccumulation Factor of the Selected Heavy Metals in the Three Vegetable Species
The trend of BF in the three selected vegetables exhibits differences in the accumulation of HMs within roots and edible parts from ambient soil.
L. siceraria
In the roots of L. siceraria, the BF trend in 2016 is Cd > Hg > Pb > Cr in monsoon and postmonsoon, but during premonsoon, a variation in trend was found (Hg > Cd > Pb > Cr). In 2017, the trend is Cd > Hg > Pb > Cr in monsoon, whereas in premonsoon and postmonsoon, the trend is Hg > Cd > Pb > Cr. In the edible parts of L. siceraria, the BF trend in 2016 is Cd > Pb > Hg > Cr throughout all the seasons, and the same trend was found in 2017 during the monsoon. However, in 2017, the trend during premonsoon and postmonsoon is Hg > Cd > Pb > Cr and Cd > Hg > Pb > Cr, respectively.
A. esculentus
In A. esculentus, the BF trend in roots is Cd > Pb > Hg > Cr during monsoon and postmonsoon in 2016, but the variation in trend during the premonsoon season was observed in the order Cd > Hg > Pb > Cr. In 2017, the trend of BF in roots is Cd > Pb > Hg > Cr during premonsoon and monsoon, whereas during postmonsoon, the trend is Cd > Hg > Pb > Cr. The BF trend in the edible parts of A. esculentus is Cd > Pb > Hg > Cr during monsoon and postmonsoon in both the years and Cd > Pb > Cr > Hg during premonsoon of both years, where Hg level remains undetected.
Z. mays
In Z. mays, the BF trend in roots is Cd > Hg > Pb > Cr through all seasons in 2016 and 2017. The trend of BF in the edible parts of Z. mays is Cd > Pb > Hg > Cr through all seasons in both the years except during monsoon in 2017, when the trend was observed to be in the sequence Cd > Hg > Pb > Cr. The BF of the three food crop species is represented in Fig. 8a and b for 2016 and 2017, respectively.
Translocation Factor of the Selected Heavy Metals in the Three Vegetable Species
A pattern of HM translocation from roots to the aerial parts needs to be established in growing plant species, which could benefit the biological monitoring of HM contamination. With BF alone, it is impossible to establish that the vegetables studied are accumulator species for certain metals with underlying health implications. The TF for L. siceraria showed the trend Pb > Cd > Cr > Hg for 2016 and 2017 through all seasons. A. esculentus and Z. mays also exhibited a similar trend for both years through all seasons (Fig. 9a and b). It is noteworthy that the TF values were found to be lower than the BF values in all selected plant species in both the years for all the HMs.
Analysis of Variance of Heavy Metals in the Roots and Edible Plant Parts of the Selected Vegetables Between Seasons and Species for 2016 and 2017
The ANOVA results exhibit significant variations of Cd, Cr, Pb, and Hg in the roots and edible parts between species and seasons, with a few exceptions (Table 1). In 2016, it was noticed that the variation of Cr and Hg in the roots between seasons is insignificant. A similar trend was observed for the seasonal variation of Hg in the roots in 2017. Additionally, in the year 2017, the variation of Hg in the edible parts between species and seasons was found to be insignificant.
Correlation Between Heavy Metal Concentrations in Soil, Roots, and Edible Parts of the Three Selected Food Crop Species
With only one exception (L. sicerariaSoil × roots), significant negative correlation coefficient values are observed between the HMs in soil and vegetables (Table 2), representing efficient uptake of the metals from the underlying soil substratum. This can be avoided by sprinkling 5% lime [Ca(OH)2] on the soil surface, thereby increasing the soil pH and preventing the transference of metals from the soil to the vegetables.
Discussion
The importance of a balanced diet has been emphasized since time immemorial. However, it has become more relevant presently when the entire humanity is grappling with the COVID-19 (coronavirus disease 2019) pandemic [40], and plant-based foods are popular among populations worldwide because of their rich nutrient, antioxidant, phytochemical, and metabolite (viz., vitamins, phytoenzymes) content [41]. In addition, they help neutralize the acidity caused by some substances aiding in digestion [42]. The increasing population worldwide has escalated the use of pesticides (herbicides, insecticides, etc.) and inorganic fertilizers in farming practices, which has impacted the health benefits and quality of the farm produce, along with the surrounding environment [43]. During their growth and development, the plants derive water and essential elements (including micro- and macronutrients) through the soil, and the contaminating HMs present in the rhizosphere soil are also taken up by the plant root systems together with these nutrients. These toxic HMs bioaccumulate in the vegetative parts and translocate from belowground biomass (roots) to aboveground biomass (stems and leaves) and eventually to the edible reproductive parts (fruits) [44]. Upon consumption of such HM-contaminated vegetables, various psychological and clinical symptoms are seen in both animals and humans that may ultimately lead to acute and/or chronic metal-induced toxicity [45].
Moreover, HM toxicity adversely impacts plant health, retards plant growth, and reduces crop yield, as reviewed extensively by Sathya et al. [7]. The study site, EKW, serves as one of the major food baskets of Kolkata and adjoining areas. The selected vegetables used in the present study are usually popular among people of all ranks of the society and hence consumed by this sizeable metropolitan population. It is the right of every individual to get access to safe food products to ensure their good health and well-being. Therefore, monitoring toxic substances in food crops, including HMs, is crucial because these toxicants tend to biomagnify in the food chain, causing severe threats to millions of people. The present work would help provide risk assessment data for the end-users in the current geographical locale, and such types of studies need to be carried out regularly to get an overall status of HM contamination in the environment and plants.
Based on previous works on EKW, it has been noticed that the four selected HMs, viz., Pb, Cd, Cr, and Hg, investigated in the present study are abundant in this unique Ramsar site [46,47,48]. The concentrations of the selected HMs in the ambient soil samples were found to be maximum in premonsoon and minimum in monsoon. The minimum value of HMs during monsoon may be attributed to two factors, namely (a) washing of the topsoil due to surface run-off caused by heavy precipitation, and (b) mixing of the study site soil with the soils from relatively uncontaminated areas around through run-offs.
We have found that all three selected vegetable species take up HMs selectively from the ambient soil, mainly during the monsoon season. The BF values show seasonal variation, with the highest value obtained during monsoon, followed by postmonsoon and premonsoon, respectively. A similar trend in TF values was observed with the exception of Hg for Z. mays in 2016 and L. siceraria in 2017, with lower values during monsoon than postmonsoon. It was observed that Pb maximally translocated from the belowground biomass to the edible parts of the plants irrespective of the species in the study area, whereas the translocation of Hg was found to be minimum. The species Z. mays showed maximum TF values for Pb for both the years through all seasons. However, a clear trend in TF for Cd, Cr, and Hg was not observed in Z. mays and A. esculentus. L. siceraria showed minimum translocation among the selected vegetable species. Compared to HM bioaccumulation, translocation of HMs from the roots into the aboveground plant tissues might be less due to the sequestration of metal ions inside the root vacuoles of the plants where HMs are fixed along with other essential elements [49]. Moreover, TF is, in turn, dependent on the pattern of HM bioaccumulation. In addition, bioaccumulation of metals in plant species varies from metal to metal and from species to species [50]. Furthermore, it has been documented by many researchers that there exists a wide variation in HM accumulation and translocation/distribution in vegetable species, which is regulated by the uptake capacity of the species, their specific parts, plant genotypes, metal types, environmental factors, edaphic factors (soil pH, soil temperature, etc.), root exudates, metal bioavailability, and anthropogenic activities [32,33,34, 51,52,53,54,55]. It can also be stated that although the amount of Pb present in the site soil was relatively less, the bioaccumulated Pb in food crops was more compared to Cd. This might either be attributed to the selective nature of Pb accumulation by these food crop species or most likely could be due to the atmospheric pollution fallout of Pb, its precipitation on the plant surfaces, and subsequent diffusion into the edible parts [56]. Since the present study area (i.e., Dhapa within EKW) is located near the megacity of Kolkata, and the busy roadway of the Eastern Metropolitan Bypass or EM Bypass (a 32-km-long major road on the east side of Kolkata) runs parallel to this site, it is pretty natural that vehicular emission of Pb from alkyl-leaded petrol or fuel with tetraethyl lead additive coupled with industrial emission of Pb from the bordering leather processing industries [57] causes Pb to concentrate in the ambient atmosphere and its deposition on the plant surfaces through atmospheric pollution fallout. In general, the monsoon seems to be highly vulnerable in terms of bioaccumulation of HMs as both BF and TF were highest in this season irrespective of the food crop species; hence there is a demand for careful monitoring along with proper blanching (soaking in warm water), washing, and cooking before consuming these vegetables.
The study site Dhapa has been used since 1879 to cultivate vegetables and has been a selected dumping ground for Kolkata city’s MSWs. Being an MSW dumping ground, it is very fertile. The sewage water generated from in and around Kolkata flows through the channels and is directed to fish ponds to culture endemic fish species and is also used for agriculture purposes. The livelihoods of many local people (around 20,000 families) depend on EKW for their sustenance by cultivating cereals (rice), vegetables, and fishes [58, 59]. In addition to the ongoing use of domestic sewage/wastewater, illegal construction, cremation, solid waste incineration, plastic recycling, and leather processing units in and around the wetlands are serious threats to this peri-urban facility, along with the HM toxicants from some of these point sources, which eventually find their way into this unique natural ecosystem. Since several toxic HMs are found in the Dhapa soil, it is regarded as metal-infested land. However, unlike other metal-impacted abandoned sites, it is very fertile because apart from the MSWs, wastewater from nearby sewage canals also reaches the soil, thereby boosting vegetation and crop cultivation. Besides the organic and inorganic contaminants, the soil also contains several essential nutrients, such as nitrates and phosphates, which promote plant growth. Therefore, the local farmers have chosen this land for growing vegetables. Owing to its proximity to the busy township of Kolkata, the produce (vegetables) from Dhapa farmland is often sold near the roadside by the local vendors; this is a common sight along the EM Bypass. Hence the farm produce reaches many households, as people stop their vehicles and purchase the products from these sellers because they are farm fresh. Upon reaching the local markets, these vegetables will be eventually bought and consumed by the populace of Kolkata.
The study results have shed light on the presence of toxic HMs in the vegetables cultivated at the study site, even though they are at lower concentrations in the reproductive parts than in the roots and soil. Nevertheless, still, it is not advisable to eat fruits/vegetables grown in a metal-contaminated land. Since cultivation is vital for the sustenance of livelihood for many farmers, it would be impossible to stop them from using the plot to grow and sell the produce on the roadside/local markets. Therefore, the need of the hour is to remediate this metal-impacted soil by co-cultivating with some metal-accumulating perennial grassy species. This will lessen the adverse impact of toxic HMs on humans as these perennial grasses will eventually accumulate more metals than the cultivable food crop species; therefore, fewer metals would be taken up by the food crops. Thus, this can be a practical roadmap to ecorestore the soil of EKW, the designated Ramsar site of West Bengal, Kolkata.
As evident from Table 3, the selected HM content in both the soil samples and the edible parts is higher than the recommended levels by the World Health Organization (WHO)/Food and Agriculture Organization (FAO) [60,61,62,63]. This requires immediate remedial measures and constant environmental monitoring for the ecorestoration of EKW as this wetland system is a unique natural ecosystem that provides not only various non-consumptive services, including biodiversity preservation, biopurification, and Kolkata’s natural drainage system, but also consumptive services, such as endemic fishes, fruits, and vegetables. The edible vegetables with HM toxicants within their body tissues can cause adverse effects in humans like central nervous system (CNS) damage, respiratory disorders, renal dysfunction, cancer, bone defects, and psychological problems [7].
Conclusions
As stated here, we can conclude some of the key findings from the present research work.
-
1.
All three selected (commonly cultivated) vegetable species of EKW, namely L. siceraria, A. esculentus, and Z. mays, selectively uptake toxic HMs from the surrounding soil. The order of HM bioaccumulation in the roots and edible parts of three food crop species is Pb > Cd > Cr > Hg.
-
2.
The biologically available HMs in the ambient soil followed the sequence Cr > Pb > Cd > Hg, which is not in sync with the accumulated HMs in the roots and edible parts of the vegetable species.
-
3.
Cr content was found to be maximum in soil samples, followed by roots and edible parts in all plant species. The soil samples contained less Pb and Cd than the roots and edible parts of plants irrespective of selected species, proving that there are more anthropogenic sources of these two HMs other than HM contamination in the soil. The concentration of Hg in soil samples was more only in A. esculentus than in the other two vegetable species.
-
4.
Moreover, HM concentration (in soil, roots, and edible parts) shows striking seasonal variations with maximum accumulation occurring in monsoon, followed by postmonsoon and premonsoon. Monsoon poses a significant threat in terms of bioaccumulation and translocation of HMs since both BF and TF were highest in this season regardless of the vegetable species, with some exceptions, i.e., TF values of Hg in 2016 for Z. mays and 2017 for L. siceraria when the monsoon values were less than postmonsoon. With the selected plant species being part of the daily diet of the local population of the district of Kolkata and adjoining districts of the state of West Bengal (due to their nutrient content, pleasant taste, affordability, and availability), it is therefore important to monitor the HM content in the vegetative and reproductive plant parts from the human health perspective. It should be noted that the vegetable species with less TF should be selected for sale and cooking purposes as it indicates lower bioavailability of the HMs to the edible parts.
To summarize, the overall result suggests proper environmental monitoring and ecorestoration of the study site, along with a few proposed preventive measures, such as careful washing and cooking while consuming these vegetables.
Future Perspectives
In keeping with the recent trend of conservative development in and around EKW, the authors view co-cultivating with low-metal-accumulating, deep-rooted, high-biomass-yielding, bioenergy-producing perennial grass species and selective PGPR strains can assist in HM phytoremediation [36, 64,65,66]. Therefore, this combinatorial approach will make the cultivable produce from the selected study site safe for human consumption besides restoring the soil ecosystem of this metal-impacted site from the perils of HM toxicity, as illustrated in detail in Fig. 10. For example, Kans grass (Saccharum spontaneum) [67,68,69,70], tall perennial grass with spreading rhizomatous roots native to the Indian subcontinent, can be used that is capable of ethanol and biogas production [71, 72]. Alternatively, ecological restoration of the metal-infested cultivable/fertile land with hyperaccumulator bioindicator species (plants with a remarkable capacity of accumulating particular HMs/metalloids within their body tissues that are hundred to thousand folds higher than other usual species of plants) [73,74,75], as listed in [76], can be done. Hyperaccumulating biodiesel/bioenergy crops with oil-rich seeds (e.g., leguminous plants like Pongamia pinnata and Brassica juncea) [77, 78] can also be applied for ecorestoration of the study site. It is noteworthy that ecorestorative studies on EKW have not been previously reported, neither PGPR-assisted ecorestorative strategies been earlier proposed by any research groups.
Multi-metallotolerant plant growth-promoting rhizobacteria (PGPR)-assisted co-cultivation of selected vegetable species with low-metal-accumulating, high-biomass-yielding, deep-rooted, bioenergy-producing perennial grass species for ecorestoration of metal-impacted, municipal solid waste dumping site of Kolkata (Dhapa) in East Kolkata Wetlands. Among the three food crop species under study, only one species (i.e., Z. mays) has been chosen for ease of representation. The use of two separate categories of PGPR strains, one for inoculating the food crop species and the other for inoculating the grassy species, is shown. It is to be noted that the graphic is not drawn to scale (created with BioRender.com)
A SWOT analysis has also been provided in Table 4 that mentions the strengths, gap areas, opportunities, and threats of the present study to implement the ecorestorative or remedial measures.
In context to phytoremediation, it is well appreciated that natural hyperaccumulator plant species are capable of phytoextracting (meaning uptake, accumulate, and transport) high concentrations of HMs; however, their slow rates of growth and limited biomass-yielding potential retard the overall rate of HM ion removal. Moreover, it is worth mentioning here that incorporating chelating substances to enhance metal bioavailability and subsequent plant-aided removal incurs additional costs and introduces new health hazards. Therefore, instead of using hyperaccumulator plants, the recently emerging phytomanagement strategy emphasizes the application of easily cultivable, low-metal-accumulating, deep-rooted, high-biomass-yielding, and biofuel/bioenergy-producing perennial grass species.
Perennial crops, including grasses and herbs, exhibit better phytoremediation traits as they show (a) fast growth, (b) high biomass yield, (c) deep and extensive fibrous root systems, (d) familiar agronomic strategies, (e) tolerance to soil contaminants, (f) greater root biomass and root surface area, and (g) tolerance to physical stresses, such as drought, soil acidity, and cold temperatures. Their inherent potential to withstand, extract, and/or stabilize HMs and their ability to yield high biomass for biofuel/bioenergy, fibers, and other commercial value-added products have generated considerable interest among managers for their implementation in several economic phytomanagement programs. Finally, besides reducing and mitigating the risks posed by the HMs, such biofuel-producing perennial grasses will provide an alternative livelihood option for the poor or low-income population of this region by opening new job markets for their products and contribute substantially to the overall economic development of the community/locality, along with meeting their energy demands through bioenergy generation [36].
For avoiding complexity, only one representative illustration has been shown for Z. mays, and the same co-cultivation procedure can be implemented for other food crop species as well (see Fig. 10). Targeted application of multiple HM-resistant and HM-bioaccumulating Halomonas and non-Halomonas PGPR strains (isolated from the rhizosphere of a true mangrove plant (Avicennia marina) of central Indian Sundarbans, West Bengal, India) [37, unpublished data] in the roots or rhizosphere soils of these routinely cultivated, dietary food crops using a biotechnological approach will facilitate plant health and nutrition besides bioremediation of toxic HMs and limiting metal uptake in food crops. This is because the perennial grass species will otherwise compete with the food crop species for available soil nutrients, negatively affecting their growth vigor, whereas the low-metal-accumulator, high-biomass-yielding, bioenergy-producing grass species will phytoextract HMs from the metal-infested ambient soil of the Dhapa site, which is otherwise used as a dumping ground of MSWs from in and around the metropolitan city of Kolkata. In contrast, targeted application of multiple HM-resistant PGPR strains (viz., Bacillus anthracis strain MHR2 (KT238975.1), Staphylococcus sp. strain MHR3 (KT238976.1), and Bacillus sp. strain MHR4 (KT238977.1)) isolated from the rhizosphere of Kans grass growing in the abandoned fly ash ponds of Mejia Thermal Power Station (MTPS), West Bengal, India (Table 5) [80], in the roots/rhizosphere soils of these grassy species (mentioned above) will facilitate enhanced HM uptake in these low-metal-accumulating, high-biomass-yielding species. It is already established in the field that the concentration of HM(s) is usually more in the belowground biomass and less in the aerial parts (and least in the fruits and seeds) [81]. However, the fruits or vegetables from these metal-incorporated food crops must be carefully soaked and washed in water before cooking and/or consumption. From the phytomanagement/phytodisposal perspective, the aboveground biomass and belowground biomass of the perennial grass species must be disposed of properly or used strategically for biodiesel extraction, following the possibility of HM recovery and recycling to be used in other industries.
Data Availability
The authors will provide the primary data if and whenever required.
References
Lokeshwari, H., & Chandrappa, G. T. (2006). Impact of heavy metal contamination of Bellandur Lake on soil and cultivated vegetation. Current Science, 91(5), 622–627.
Oliver, M. A. (1997). Soil and human health: a review. European Journal of Soil Science, 48(4), 573–592. https://doi.org/10.1111/j.1365-2389.1997.tb00558.x
Sharma, R. K., Agrawal, M., & Marshall, F. M. (2008). Heavy metal (Cu, Zn, Cd and Pb) contamination of vegetables in urban India: a case study in Varanasi. Environmental Pollution, 154(2), 254–263. https://doi.org/10.1016/j.envpol.2007.10.010
Csavina, J., Field, J., Taylor, M. P., Gao, S., Landázuri, A., Betterton, E. A., & Sáez, A. E. (2012). A review on the importance of metals and metalloids in atmospheric dust and aerosol from mining operations. Science of The Total Environment, 433, 58–73. https://doi.org/10.1016/j.scitotenv.2012.06.013
Morais, S., e Costa, F. G., & Pereira, M. d. L. (2012). Heavy metals and human health. In J. Oosthuizen (Ed.), Environmental Health – Emerging Issues and Practice (pp. 227–246). IntechOpen Limited. https://doi.org/10.5772/29869
Sharma, B., Singh, S., & Siddiqi, N. J. (2014). Biomedical implications of heavy metals induced imbalances in redox systems. BioMed Research International, 2014(640754), 1–26. https://doi.org/10.1155/2014/640754
Sathya, A., Kanaganahalli, V., Rao, P. S., & Gopalakrishnan, S. (2016). Cultivation of sweet Sorghum on heavy metal-contaminated soils by phytoremediation approach for production of bioethanol. In M. N. V. Prasad (Ed.), Bioremediation and Bioeconomy (pp. 271–292). Elsevier. https://doi.org/10.1016/B978-0-12-802830-8.00012-5
United States Environmental Protection Agency, USEPA. (2000). Electrokinetic and phytoremediation in situ treatment of metal-contaminated soil: State-of-the-practice. Draft for Final Review. EPA/542/R-00/XXX. US Environmental Protection Agency, Office of Solid Waste and Emergency Response Technology Innovation Office, Washington DC, USA.
Boran, M., & Altinok, I. (2010). A review of heavy metals in water, sediment and living organisms in the black sea. Turkish Journal of Fisheries and Aquatic Sciences, 10, 565–572. https://doi.org/10.4194/trjfas.2010.0418
Bhattacharya, S., Ganguli, A., Bose, S., & Mukhopadhyay, A. (2012). Biodiversity, traditional practices and sustainability issues of East Kolkata Wetlands: A significance Ramsar site of West Bengal, (India). Research & Reviews in BioSciences, 6(11), 340–347.
Kundu, N., Pal, M., & Saha, S. (2008). East Kolkata Wetlands: A resource recovery system through productive activities. In M. Sengupta, & R. Dalwani, (Eds.), Proceedings of Taal2007: The 12th World Lake Conference (pp. 868–881).
https://sandrp.in/2020/03/07/india-ramsar-wetlands-in-crisis-in-2020
Dey, D., & Banerjee, S. (2013). Ecosystem and livelihood support: The story of East Kolkata Wetlands. Environment and Urbanization ASIA, 4(2), 325–337. https://doi.org/10.1177/0975425313511158
Dutta, J., Zaman, S., Thakur, T. K., Kaushik, S., Mitra, A., Singh, P., Kumar, R., Zuan, A. T. K., Samdani, M. S., Alharbi, S. A., & Datta, R. (2022). Assessment of the bioaccumulation pattern of Pb, Cd, Cr and Hg in edible fishes of East Kolkata Wetlands, India. Saudi Journal of Biological Sciences, 29(2), 758–766. https://doi.org/10.1016/j.sjbs.2021.09.039
Ray Chaudhuri, S., Mukherjee, I., Ghosh, D., & Thakur, A. R. (2012). East Kolkata Wetland: A multifunctional niche of international importance. OnLine Journal of Biological Sciences, 12(2), 80–88. https://doi.org/10.3844/ojbsci.2012.80.88
Barot, A., Pinto, S., Balakrishnan, S., & Prajapati, J. P. (2015). Composition, functional properties and application of bottle gourd in food products. Research & Reviews: Journal of Dairy Science and Technology, 4(1), 15–27. https://doi.org/10.37591/rrjodst.v4i1.407
Elkhalifa, A. E. O., Alshammari, E., Adnan, M., Alcantara, J. C., Awadelkareem, A. M., Eltoum, N. E., Mehmood, K., Panda, B. P., & Ashraf, S. A. (2021). Okra (Abelmoschus Esculentus) as a potential dietary medicine with nutraceutical importance for sustainable health applications. Molecules, 26(3), 696. https://doi.org/10.3390/molecules26030696
Lyon, F. (2000). Trust, networks and norms: The creation of social capital in agricultural economies in Ghana. World Development, 28(4), 663–681. https://doi.org/10.1016/S0305-750X(99)00146-1
Nwosu, L. C. (2018). Maize and the maize weevil: advances and innovations in postharvest control of the pest. Food Quality and Safety, 2(3), 145–152. https://doi.org/10.1093/fqsafe/fyy011
Dias, J. S. (2012). Nutritional quality and health benefits of vegetables: A review. Food and Nutrition Sciences, 3(10), 1354–1374. https://doi.org/10.4236/fns.2012.310179
Radwan, M. A., & Salama, A. K. (2006). Market basket survey for some heavy metals in Egyptian fruits and vegetables. Food and Chemical Toxicology, 44(8), 1273–1278. https://doi.org/10.1016/j.fct.2006.02.004
Khan, S., Cao, Q., Zheng, Y. M., Huang, Y. Z., & Zhu, Y. G. (2008). Health risks of heavy metals in contaminated soils and food crops irrigated with wastewater in Beijing, China. Environmental Pollution, 152(3), 686–692. https://doi.org/10.1016/j.envpol.2007.06.056
Salido, A. L., Hasty, K. L., Lim, J.-M., & Butcher, D. J. (2003). Phytoremediation of arsenic and lead in contaminated soil using Chinese brake ferns (Pteris vittata) and Indian mustard (Brassica juncea). International Journal of Phytoremediation, 5(2), 89–103. https://doi.org/10.1080/713610173
Erdei, L., Mezösi, G., Mécs, I., Vass, I., Föglein, F., & Bulik, L. (2005). Phytoremediation as a program for decontamination of heavy-metal polluted environment. Acta Biologica Szegediensis, 49(1–2), 75–76.
Al Jassir, M., Shaker, A., & Khaliq, M. (2005). Deposition of heavy metals on green leafy vegetables sold on roadsides of Riyadh City, Saudi Arabia. Bulletin of Environmental Contamination and Toxicology, 75, 1020–1027. https://doi.org/10.1007/s00128-005-0851-4
Arora, M., Kiran, B., Rani, S., Rani, A., Kaur, B., & Mittal, N. (2008). Heavy metal accumulation in vegetables irrigated with water from different sources. Food Chemistry, 111(4), 811–815. https://doi.org/10.1016/j.foodchem.2008.04.049
Sipter, E., Rózsa, E., Gruiz, K., Tátrai, E., & Morvai, V. (2008). Site-specific risk assessment in contaminated vegetable gardens. Chemosphere, 71(7), 1301–1307. https://doi.org/10.1016/j.chemosphere.2007.11.039
Singh, S., Zacharias, M., Kalpana, S., & Mishra, S. (2012). Heavy metals accumulation and distribution pattern in different vegetable crops. Journal of Environmental Chemistry and Ecotoxicology, 4(10), 170–177. https://doi.org/10.5897/JECE11.076
Radulescu, C., Stihi, C., Barbes, L., Chilian, A., & Chelarescu, D. E. (2013). Studies concerning heavy metals accumulation of carduus nutans l. and taraxacum officinale as potential soil bioindicator species. Revista de Chimie, 64(7), 754–760
Radulescu, C., Stihi, C., Busuioc, G., Gheboianu, A. I., & Popescu, I. V. (2010). Studies concerning heavy metals bioaccumulation of wild edible mushrooms from industrial area by using spectrometric techniques. Bulletin of Environmental Contamination and Toxicology, 84, 641–646. https://doi.org/10.1007/s00128-010-9976-1
Dulama, I., Popescu, I. V., Stihi, C., Radulescu, C., Cimpoca, G. V., Toma, L. G., Stirbescu, R., & Nitescu, O. (2012). Studies on accumulation of heavy metals in Acacia leaf by EDXRF. Romanian Reports in Physics, 64(4), 1063–1071
Ye, X., Xiao, W., Zhang, Y., Zhao, S., Wang, G., Zhang, Q., & Wang, Q. (2015). Assessment of heavy metal pollution in vegetables and relationships with soil heavy metal distribution in Zhejiang province, China. Environmental Monitoring and Assessment, 187, 378. https://doi.org/10.1007/s10661-015-4604-5
Roy, M., & Pandey, V. C. (2020). Role of microbes in grass-based phytoremediation. In V. C. Pandey, & D. P. Singh (Eds.), Phytoremediation Potential of Perennial Grasses (pp. 303–336). Elsevier.
Mukherjee, P., Mitra, A., & Roy, M. (2019). Halomonas rhizobacteria of avicennia marina of Indian sundarbans promote rice growth under saline and heavy metal stresses through exopolysaccharide production. Frontiers in Microbiology, 10, 1207. https://doi.org/10.3389/fmicb.2019.01207
Kumar, S., Singh, J., Das, S., & Garg, M. (2012). AAS estimation of heavy metals and trace elements in Indian herbal cosmetic preparations. Research Journal of Chemical Sciences, 2(3), 46–51.
Malo, B. A. (1977). Partial extraction of metals from aquatic sediments. Environmental Science and Technology, 11(3), 277–282. https://doi.org/10.1021/es60126a007
Agarwal, S., Saha, S., Deb, T., & Darbar, S. (2020). Immunity augmenting food supplements for susceptible individuals in combating pandemic COVID-19 (Review). Parana Journal of Science and Education (PJSE), 6(4), 79–88. https://doi.org/10.5281/zenodo.3880638
Agarwal, S., Darbar, S., Saha, S., & Deb, T. (2021). Prevention is always better than cure: Immunity boosting to fight infections. Open Journal of Medical Sciences, 1, 28–42. https://doi.org/10.31586/ojms.2021.010104
Li, T. S. C. (2008). Vegetables and fruits: Nutritional and therapeutic values. CRC Press. https://doi.org/10.1201/9781420068733
Tilman, D., Fargione, J., Wolff, B., D’Antonio, C., Dobson, A., Howarth, R., Schindler, D., Schlesinger, W. H., Simberloff, D., & Swackhamer, D. (2001). Forecasting agriculturally driven global environmental change. Science, 292(5515), 281–284. https://doi.org/10.1126/science.1057544
Bahemuka, T. E., & Mubofu, E. B. (1999). Heavy metals in edible green vegetables grown along the sites of the Sinza and Msimbazi rivers in Dar es Salaam, Tanzania. Food Chemistry, 66(1), 63–66. https://doi.org/10.1016/S0308-8146(98)00213-1
Alam, M. G. M., Snow, E. T., & Tanaka, A. (2003). Arsenic and heavy metal contamination of vegetables grown in Samta village, Bangladesh. Science of The Total Environment, 308(1-3), 83–96. https://doi.org/10.1016/S0048-9697(02)00651-4
Dutta, J., Zaman, S., & Mitra, A. (2017). Bioaccumulation of toxic heavy metals in some commonly edible vegetables grown in East Kolkata Wetland (EKW), the designated ramsar site of West Bengal, India. Journal of Science, Engineering, Health and Management, 1(4), 55–58.
Dutta, J., Zaman, S., & Mitra, A. (2019). Are we taking poison every day? Indian Journal of Environmental Protection, 39(3), 283–289.
Dutta, J., Choudhary, G. R., Mitra, A. (2017). Bioaccumulation of toxic heavy metals in the edible fishes of Eastern Kolkata Wetlands (EKW), the designated ramsar site of West Bengal, India. International Journal of Aquaculture and Fishery Sciences, 3(1), 018–021. https://doi.org/10.17352/2455-8400.000023
Shanker, A. K., Cervantes, C., Loza-Tavera, H., & Avudainayagam, S. (2005). Chromium toxicity in plants. Environment International, 31(5), 739–753. https://doi.org/10.1016/j.envint.2005.02.003
Baker, A. J. M. (1981). Accumulators and excluders -strategies in the response of plants to heavy metals. Journal of Plant Nutrition, 3(1-4), 643–654. https://doi.org/10.1080/01904168109362867
McLaughlin, M. J., Parker, D. R., & Clarke, J. M. (1999). Metals and micronutrients – food safety issues. Field Crops Research, 60(1-2), 143–163. https://doi.org/10.1016/S0378-4290(98)00137-3
Zhu, Y., Yu, H., Wang, J., Fang, W., Yuan, J., & Yang, Z. (2007). Heavy metal accumulations of 24 asparagus bean cultivars grown in soil contaminated with Cd alone and with multiple metals (Cd, Pb, and Zn). Journal of Agricultural and Food Chemistry, 55(3), 1045–1052. https://doi.org/10.1021/jf062971p
Säumel, I., Kotsyuk, I., Hölscher, M., Lenkereit, C., Weber, F., & Kowarik, I. (2012). How healthy is urban horticulture in high traffic areas? Trace metal concentrations in vegetable crops from plantings within inner city neighbourhoods in Berlin, Germany. Environmental Pollution, 165, 124–132. https://doi.org/10.1016/j.envpol.2012.02.019
Qadir, S., Jamshieed, S., Rasool, S., Ashraf, M., Akram, N. A., & Ahmad, P. (2014). Modulation of plant growth and metabolism in cadmium-enriched environments. In Whitacre, D. (ed.), Reviews of Environmental Contamination and Toxicology (pp. 51–88). Springer. https://doi.org/10.1007/978-3-319-03777-6_4
Zhou, H., Yang, W.-T., Zhou, X., Liu, L., Gu, J.-F., Wang, W.-L., Zou, J.-L., Tian, T., Peng, P.-Q., & Liao, B.-H. (2016). Accumulation of heavy metals in vegetable species planted in contaminated soils and the health risk assessment. International Journal of Environmental Research and Public Health, 13(3), 289. https://doi.org/10.3390/ijerph13030289
Renberg, I., Bindler, R., & Brännvall, M.-L. (2001). Using the historical atmospheric lead-deposition record as a chronological marker in sediment deposits in Europe. The Holocene, 11(5), 511–516. https://doi.org/10.1191/095968301680223468
Chowdhury, M., Mostafa, M. G., Biswas, T. K., Mandal, A., & Saha, A. K. (2015). Characterization of the effluents from leather processing industries. Environmental Processes, 2, 173–187. https://doi.org/10.1007/s40710-015-0065-7
Bunting, S. W., Kundu, N., Punch, S., & Little, D. C. (2001). East Kolkata wetlands and livelihoods: Workshop proceedings. Working paper 2. Institute of Aquaculture, Stirling, UK.
Bunting, S. W., Kundu, N., & Mukherjee, M. (2001). Literature review: Renewable natural resource-use in livelihoods at the Calcutta peri-urban interface. Working paper 1. Institute of Aquaculture, Stirling, UK.
Joint FAO/WHO Expert Committee on Food Additives, Food and Agriculture Organization of the United Nations & World Health Organization. (1989). Evaluation of certain food additives and contaminants: thirty-third report of the joint FAO/WHO expert committee on food additives, 21-30 March 1988. World Health Organization, Geneva. https://apps.who.int/iris/handle/10665/39252
Joint FAO/WHO Expert Committee on Food Additives, World Health Organization & Food and Agriculture Organization of the United Nations. (1993). Evaluation of certain food additives and contaminants: forty-first report of the joint FAO/WHO expert committee on food additives, 9-18 February 1993. World Health Organization, Geneva. https://apps.who.int/iris/handle/10665/36981
Joint FAO/WHO Expert Committee on Food Additives, World Health Organization & Food and Agriculture Organization of the United Nations. (1989). Toxicological evaluation of certain food additives and contaminants / prepared by the 33rd meeting of the joint FAO/WHO expert committee on food additives, Geneva, 21-30 March 1989. Cambridge University Press, Cambridge. https://apps.who.int/iris/handle/10665/41268
FAO/WHO. (2011). Codex alimentarius commission. Joint FAO/WHO food standards programme codex committee on contaminants in foods, fifth session, 21-25 March 2011. Working document for information and use in discussions related to contaminants and toxins in the GSCTFF (Prepared by Japan and the Netherlands) CF/5 INF/1. The Hague, The Netherlands.
Datta, U., Zaman, S., & Mitra, A. (2017). Enrichment factor and translocation factor of selective heavy metals in Saccharum spontaneum and Typha elephantina species growing in the fly ash ponds of Mejia Thermal Power Station (MTPS). Journal of Science, Engineering, Health and Management, 1(2), 40–44.
Roy, M., Giri, A. K., Dutta, S., & Mukherjee, P. (2015). Integrated phytobial remediation for sustainable management of arsenic in soil and water. Environment International, 75, 180–198. https://doi.org/10.1016/j.envint.2014.11.010
Khodijah, N. S., Suwignyo, R. A., Harun, M. U., & Robiartini, L. (2019). Phytoremediation potential of some grasses on lead heavy metal in tailing planting media of former tin mining. Biodiversitas Journal of Biological Diversity, 20(7), 1973–1982. https://doi.org/10.13057/biodiv/d200725
Pandey, V. C., & Singh, N. (2014). Fast green capping on coal fly ash basins through ecological engineering. Ecological Engineering, 73, 671–675. https://doi.org/10.1016/j.ecoleng.2014.09.036
Kumar, A., Ahirwal, J., Maiti, S. K., & Das, R. (2015). An assessment of metal in fly ash and their translocation and bioaccumulation in perennial grasses growing at the reclaimed opencast mines. International Journal of Environmental Research, 9(3), 1089–1096. https://doi.org/10.22059/IJER.2015.996
Pandey, V. C., Bajpai, O., Pandey, D. N., & Singh, N. (2015). Saccharum spontaneum: an underutilized tall grass for revegetation and restoration programs. Genetic Resources and Crop Evolution, 62, 443–450. https://doi.org/10.1007/s10722-014-0208-0
Pandey, V. C., Prakash, P., Bajpai, O., Kumar, A., & Singh, N. (2015). Phytodiversity on fly ash deposits: evaluation of naturally colonized species for sustainable phytorestoration. Environmental Science and Pollution Research, 22, 2776–2787. https://doi.org/10.1007/s11356-014-3517-0
Bhandari, M. M., & Vyas, S. P. (1990). Flora of the Indian Desert. MPS Repros. https://doi.org/10.1017/S0960428600003103
Chandel, A. K., Narasu, M. L., Chandrasekhar, G., Manikyam, A., & Rao, L. V. (2009). Use of Saccharum spontaneum (wild sugarcane) as biomaterial for cell immobilization and modulated ethanol production by thermotolerant Saccharomyces cerevisiae VS3. Bioresource Technology, 100(8), 2404–2410. https://doi.org/10.1016/j.biortech.2008.11.014
Reeves, R. D. (2003). Tropical hyperaccumulators of metals and their potential for phytoextraction. Plant and Soil, 249, 57–65.
van der Ent, A., Baker, A. J. M., Reeves, R. D., Pollard, A. J., & Schat, H. (2013). Hyperaccumulators of metal and metalloid trace elements: Facts and fiction. Plant and Soil, 362, 319–334. https://doi.org/10.1007/s11104-012-1287-3
Reeves, R. D., Baker, A. J. M., Jaffré, T., Erskine, P. D., Echevarria, G., & van der Ent, A. (2017). A global database for plants that hyperaccumulate metal and metalloid trace elements. New Phytologist, 218(2), 407–411. https://doi.org/10.1111/nph.14907
Gresshoff, P. M., Rangan, L., Indrasumunar, A., & Scott, P. T. (2017). A new bioenergy crop based on oil-rich seeds from the legume tree Pongamia pinnata. Energy and Emission Control Technologies, 5, 19–26. https://doi.org/10.2147/EECT.S69854
Małecka, A., Konkolewska, A., Hanć, A., Barałkiewicz, D., Ciszewska, L., Ratajczak, E., Staszak, A. M., Kmita, H., & Jarmuszkiewicz, W. (2019). Insight into the phytoremediation capability of Brassica juncea (v. Malopolska): Metal accumulation and antioxidant enzyme activity. International Journal of Molecular Sciences, 20(18), 4355. https://doi.org/10.3390/ijms20184355
Thakur, T. K., Dutta, J., Upadhyay, P., Patel, D. K., Thakur, A., Kumar, M., & Kumar, A. (2022). Assessment of land degradation and restoration in coal mines of central India: A time series analysis. Ecological Engineering, 175, 106493. https://doi.org/10.1016/j.ecoleng.2021.106493
Mukherjee, P., Roychowdhury, R., & Roy, M. (2017). Phytoremediation potential of rhizobacterial isolates from Kans grass (Saccharum spontaneum) of fly ash ponds. Clean Technologies and Environmental Policy, 19, 1373–1385. https://doi.org/10.1007/s10098-017-1336-y
Mukherjee, P., Pramanick, P., Zaman, S., & Mitra, A. (2021). Phytoremediation of heavy metals by the dominant mangrove associate species of Indian Sundarbans. Journal of Environmental Engineering and Landscape Management, 29(4), 391–402. https://doi.org/10.3846/jeelm.2021.15773
Acknowledgements
The authors are thankful to Techno India University, West Bengal, for providing the necessary instruments, equipment, and reagents for carrying out the entire research work. The authors are grateful to the RCC Institute of Information Technology (RCCIIT) staff and management for extending all possible support in conducting the present study.
Author information
Authors and Affiliations
Contributions
Sangita Agarwal, Pritam Mukherjee, and Abhijit Mitra have conceived the idea, designed experiments, and done the field samplings. Pritam Mukherjee and Prosenjit Pramanick prepared the materials, while Sangita Agarwal and Pritam Mukherjee carried out the experiments. Sangita Agarwal, Pritam Mukherjee, and Abhijit Mitra have analyzed and interpreted the data. Pritam Mukherjee and Prosenjit Pramanick have made all the graphs and tables and have done the statistical analyses. Sangita Agarwal, Pritam Mukherjee, and Abhijit Mitra wrote the paper. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent to Publish
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Agarwal, S., Mukherjee, P., Pramanick, P. et al. Seasonal Variations in Bioaccumulation and Translocation of Toxic Heavy Metals in the Dominant Vegetables of East Kolkata Wetlands: a Case Study with Suggestive Ecorestorative Strategies. Appl Biochem Biotechnol 195, 2332–2358 (2023). https://doi.org/10.1007/s12010-022-04057-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-022-04057-6