Abstract
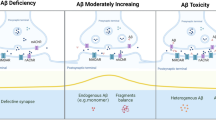
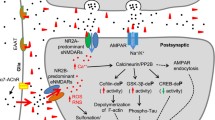
Alzheimer’s disease (AD) is characterized neuropathologically by the deposition of different forms of amyloid β-protein (Aβ) including variable amounts of soluble species that correlate with severity of dementia. The extent of synaptic loss in the brain provides the best morphological correlate of cognitive impairment in clinical AD. Animal research on the pathophysiology of AD has therefore focussed on how soluble Aβ disrupts synaptic mechanisms in vulnerable brain regions such as the hippocampus. Synapic plasticity in the form of persistent activity-dependent increases or decreases in synaptic strength provide a neurophysiological substrate for hippocampal-dependent learning and memory. Acute treatment with human-derived or chemically prepared soluble Aβ that contains certain oligomeric assemblies, potently and selectively disrupts synaptic plasticity causing inhibition of long-term potentiation (LTP) and enhancement of long-term depression (LTD) of glutamatergic transmission. Over time these and related actions of Aβ have been implicated in reducing synaptic integrity. This review addresses the involvement of neurotransmitter intercellular signaling in mediating or modulating the synaptic plasticity disrupting actions of soluble Aβ, with particular emphasis on the different roles of glutamatergic and cholinergic mechanisms. There is growing evidence to support the view that NMDA and possibly nicotinic receptors are critically involved in mediating the disruptive effect of Aβ and that targeting muscarinic receptors can indirectly modulate Aβ’s actions. Such studies should help inform ongoing and future clinical trials of drugs acting through the glutamatergic and cholinergic systems.


Similar content being viewed by others
References
Abbott, J. J., Howlett, D. R., Francis, P. T., & Williams, R. J. (2008). Abeta(1–42) modulation of Akt phosphorylation via alpha7 nAChR and NMDA receptors. Neurobiology of Aging, 29, 992–1001.
Albuquerque, E. X., Alkondon, M., Pereira, E. F., et al. (1997). Properties of neuronal nicotinic acetylcholine receptors: Pharmacological characterization and modulation of synaptic function. Journal of Pharmacology and Experimental Therapeutics, 280, 1117–1136.
Almeida, C. G., Tampellini, D., Takahashi, R. H., et al. (2005). Beta-amyloid accumulation in APP mutant neurons reduces PSD-95 and GluR1 in synapses. Neurobiology of Diseases, 20, 187–198.
Anwyl, R. (1999). Metabotropic glutamate receptors: Electrophysiological properties and role in plasticity. Brain Research. Brain Research Reviews, 29, 83–120.
Arendt, T. (2009). Synaptic degeneration in Alzheimer’s disease. Acta Neuropathologica, 118, 167–179.
Arias, C., Arrieta, I., & Tapia, R. (1995). Beta-amyloid peptide fragment 25–35 potentiates the calcium-dependent release of excitatory amino acids from depolarized hippocampal slices. Journal of Neuroscience Research, 41, 561–566.
Auld, D. S., Kornecook, T. J., Bastianetto, S., & Quirion, R. (2002). Alzheimer’s disease and the basal forebrain cholinergic system: Relations to beta-amyloid peptides, cognition, and treatment strategies. Progress in Neurobiology, 68, 209–245.
Bancroft, A., & Levin, E. D. (2000). Ventral hippocampal alpha4beta2 nicotinic receptors and chronic nicotine effects on memory. Neuropharmacology, 39, 2770–2778.
Barghorn, S., Nimmrich, V., Striebinger, A., et al. (2005). Globular amyloid beta-peptide oligomer—a homogenous and stable neuropathological protein in Alzheimer’s disease. Journal of Neurochemistry, 95, 834–847.
Bishop, G. M., & Robinson, S. R. (2004). Physiological roles of amyloid-beta and implications for its removal in Alzheimer’s disease. Drugs and Aging, 21, 621–630.
Biton, B., Bergis, O. E., Galli, F., et al. (2007). SSR180711, a novel selective alpha7 nicotinic receptor partial agonist: (1) Binding and functional profile. Neuropsychopharmacology, 32, 1–16.
Blusztajn, J. K., & Berse, B. (2000). The cholinergic neuronal phenotype in Alzheimer’s disease. Metabolic Brain Disease, 15, 45–64.
Bobich, J. A., Zheng, Q., & Campbell, A. (2004). Incubation of nerve endings with a physiological concentration of Abeta1-42 activates CaV2.2(N-Type)-voltage operated calcium channels and acutely increases glutamate and noradrenaline release. Journal of Alzheimer’s Disease, 6, 243–255.
Bodick, N. C., Offen, W. W., Levey, A. I., et al. (1997). Effects of xanomeline, a selective muscarinic receptor agonist, on cognitive function and behavioral symptoms in Alzheimer disease. Archives of Neurology, 54, 465–473.
Bourin, M., Ripoll, N., & Dailly, E. (2003). Nicotinic receptors and Alzheimer’s disease. Current Medical Research and Opinion, 19, 169–177.
Busche, M. A., Eichhoff, G., Adelsberger, H., et al. (2008). Clusters of hyperactive neurons near amyloid plaques in a mouse model of Alzheimer’s disease. Science, 321, 1686–1689.
Bymaster, F. P., Shannon, H. E., Rasmussen, K., et al. (1998). Unexpected antipsychotic-like activity with the muscarinic receptor ligand (5R, 6R)6-(3-propylthio-1, 2, 5-thiadiazol-4-yl)-1-azabicyclo[3.2.1]octane. European Journal of Pharmacology, 356, 109–119.
Cacucci, F., Yi, M., Wills, T. J., Chapman, P., & O’Keefe, J. (2008). Place cell firing correlates with memory deficits and amyloid plaque burden in Tg2576 Alzheimer mouse model. Proceedings of the National Academy of Sciences of the United States of America, 105, 7863–7868.
Chen, Q. S., Wei, W. Z., Shimahara, T., & Xie, C. W. (2002). Alzheimer amyloid beta-peptide inhibits the late phase of long-term potentiation through calcineurin-dependent mechanisms in the hippocampal dentate gyrus. Neurobiology of Learning and Memory, 77, 354–371.
Chen, L., Yamada, K., Nabeshima, T., & Sokabe, M. (2006). Alpha7 nicotinic acetylcholine receptor as a target to rescue deficit in hippocampal LTP induction in beta-amyloid infused rats. Neuropharmacology, 50, 254–268.
Cheng, L., Yin, W. J., Zhang, J. F., & Qi, J. S. (2009). Amyloid beta-protein fragments 25-35 and 31-35 potentiate long-term depression in hippocampal CA1 region of rats in vivo. Synapse, 63, 206–214.
Chin, J. H., Ma, L., MacTavish, D., & Jhamandas, J. H. (2007). Amyloid beta protein modulates glutamate-mediated neurotransmission in the rat basal forebrain: Involvement of presynaptic neuronal nicotinic acetylcholine and metabotropic glutamate receptors. Journal of Neuroscience, 27, 9262–9269.
Chishti, M. A., Yang, D. S., Janus, C., et al. (2001). Early-onset amyloid deposition and cognitive deficits in transgenic mice expressing a double mutant form of amyloid precursor protein 695. Journal of Biological Chemistry, 276, 21562–21570.
Ciccotosto, G. D., Tew, D. J., Drew, S. C., et al. (2009). Stereospecific interactions are necessary for Alzheimer disease amyloid-beta toxicity. Neurobiology of Aging. doi:10.1016/j.neurobiolaging.2009.02.018.
Cleary, J. P., Walsh, D. M., Hofmeister, J. J., et al. (2005). Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nature Neuroscience, 8, 79–84.
Coan, E. J., Irving, A. J., & Collingridge, G. L. (1989). Low-frequency activation of the NMDA receptor system can prevent the induction of LTP. Neuroscience Letters, 105, 205–210.
Court, J., Martin-Ruiz, C., Piggott, M., Spurden, D., Griffiths, M., & Perry, E. (2001). Nicotinic receptor abnormalities in Alzheimer’s disease. Biological Psychiatry, 49, 175–184.
Cowburn, R. F., Wiehager, B., Trief, E., Li-Li, M., & Sundstrom, E. (1997). Effects of beta-amyloid-(25-35) peptides on radioligand binding to excitatory amino acid receptors and voltage-dependent calcium channels: Evidence for a selective affinity for the glutamate and glycine recognition sites of the NMDA receptor. Neurochemical Research, 22, 1437–1442.
Cullen, W. K., Wu, J., Anwyl, R., & Rowan, M. J. (1996). Beta-amyloid produces a delayed NMDA receptor-dependent reduction in synaptic transmission in rat hippocampus. NeuroReport, 8, 87–92.
Cullen, W. K., Suh, Y. H., Anwyl, R., & Rowan, M. J. (1997). Block of LTP in rat hippocampus in vivo by β-amyloid precursor protein fragments. NeuroReport, 8, 3213–3217.
Dawson, G. R., Seabrook, G. R., Zheng, H., et al. (1999). Age-related cognitive deficits, impaired long-term potentiation and reduction in synaptic marker density in mice lacking the beta-amyloid precursor protein. Neuroscience, 90, 1–13.
De Felice, F. G., Velasco, P. T., Lambert, M. P., et al. (2007). Abeta oligomers induce neuronal oxidative stress through an N-methyl-D-aspartate receptor-dependent mechanism that is blocked by the Alzheimer drug memantine. Journal of Biological Chemistry, 282, 11590–11601.
Deshpande, A., Kawai, H., Metherate, R., Glabe, C. G., & Busciglio, J. (2009). A role for synaptic zinc in activity-dependent Abeta oligomer formation and accumulation at excitatory synapses. Journal of Neuroscience, 29, 4004–4015.
Dewachter, I., Filipkowski, R. K., Priller, C., et al. (2009). Deregulation of NMDA-receptor function and down-stream signaling in APP[V717I] transgenic mice. Neurobiology of Aging, 30, 241–256.
Dineley, K. T., Westerman, M., Bui, D., Bell, K., Ashe, K. H., & Sweatt, J. D. (2001). Beta-amyloid activates the mitogen-activated protein kinase cascade via hippocampal alpha7 nicotinic acetylcholine receptors: In vitro and in vivo mechanisms related to Alzheimer’s disease. Journal of Neuroscience, 21, 4125–4133.
Dineley, K. T., Bell, K. A., Bui, D., & Sweatt, J. D. (2002). Beta-amyloid peptide activates alpha 7 nicotinic acetylcholine receptors expressed in Xenopus oocytes. Journal of Biological Chemistry, 277, 25056–25061.
Domingues, A., Almeida, S., da Cruz e Silva, E. F., Oliveira, C. R., & Rego, A. C. (2007). Toxicity of beta-amyloid in HEK293 cells expressing NR1/NR2A or NR1/NR2B N-methyl-D-aspartate receptor subunits. Neurochemistry International, 50, 872–880.
Dornan, W. A., Kang, D. E., McCampbell, A., & Kang, E. E. (1993). Bilateral injections of beta A(25-35) + IBO into the hippocampus disrupts acquisition of spatial learning in the rat. NeuroReport, 5, 165–168.
Dziewczapolski, G., Glogowski, C. M., Masliah, E., & Heinemann, S. F. (2009). Deletion of the alpha7 nicotinic acetylcholine receptor gene improves cognitive deficits and synaptic pathology in a mouse model of Alzheimer’s disease. Journal of Neuroscience, 29, 8805–8815.
Ferchmin, P. A., Perez, D., Eterovic, V. A., & de Vellis, J. (2003). Nicotinic receptors differentially regulate N-methyl-D-aspartate damage in acute hippocampal slices. Journal of Pharmacology and Experimental Therapeutics, 305, 1071–1078.
Fernandez-Tome, P., Brera, B., Arevalo, M. A., & de Ceballos, M. L. (2004). Beta-amyloid25–35 inhibits glutamate uptake in cultured neurons and astrocytes: Modulation of uptake as a survival mechanism. Neurobiology of Diseases, 15, 580–589.
Fodero, L. R., Mok, S. S., Losic, D., et al. (2004). Alpha7-nicotinic acetylcholine receptors mediate an Abeta(1-42)-induced increase in the level of acetylcholinesterase in primary cortical neurones. Journal of Neurochemistry, 88, 1186–1193.
Frankiewicz, T., Potier, B., Bashir, Z. I., Collingridge, G. L., & Parsons, C. G. (1996). Effects of memantine and MK-801 on NMDA-induced currents in cultured neurones and on synaptic transmission and LTP in area CA1 of rat hippocampal slices. British Journal of Pharmacology, 117, 689–697.
Freir, D. B., & Herron, C. E. (2003). Nicotine enhances the depressive actions of A beta 1-40 on long-term potentiation in the rat hippocampal CA1 region in vivo. Journal of Neurophysiology, 89, 2917–2922.
Fujii, S., Ji, Z., Morita, N., & Sumikawa, K. (1999). Acute and chronic nicotine exposure differentially facilitate the induction of LTP. Brain Research, 846, 137–143.
Gay, E. A., Giniatullin, R., Skorinkin, A., & Yakel, J. L. (2008). Aromatic residues at position 55 of rat alpha7 nicotinic acetylcholine receptors are critical for maintaining rapid desensitization. Journal of Physiology, 586, 1105–1115.
Ge, S., & Dani, J. A. (2005). Nicotinic acetylcholine receptors at glutamate synapses facilitate long-term depression or potentiation. Journal of Neuroscience, 25, 6084–6091.
Geula, C., Nagykery, N., Nicholas, A., & Wu, C. K. (2008). Cholinergic neuronal and axonal abnormalities are present early in aging and in Alzheimer disease. Journal of Neuropathology and Experimental Neurology, 67, 309–318.
Goto, Y., Niidome, T., Hongo, H., Akaike, A., Kihara, T., & Sugimoto, H. (2008). Impaired muscarinic regulation of excitatory synaptic transmission in the APPswe/PS1dE9 mouse model of Alzheimer’s disease. European Journal of Pharmacology, 583, 84–91.
Grassi, F., Palma, E., Tonini, R., Amici, M., Ballivet, M., & Eusebi, F. (2003). Amyloid beta(1-42) peptide alters the gating of human and mouse alpha-bungarotoxin-sensitive nicotinic receptors. Journal of Physiology, 547, 147–157.
Greenamyre, J. T., & Young, A. B. (1989). Excitatory amino acids and Alzheimer’s disease. Neurobiology of Aging, 10, 593–602.
Gu, Z., Liu, W., & Yan, Z. (2009). Beta-amyloid impairs AMPA receptor trafficking and function by reducing Ca2+/calmodulin-dependent protein kinase II synaptic distribution. Journal of Biological Chemistry, 284, 10639–10649.
Haass, C., & Selkoe, D. J. (2007). Soluble protein oligomers in neurodegeneration: Lessons from the Alzheimer’s amyloid beta-peptide. Nature Reviews. Molecular Cell Biology, 8, 101–112.
Harkany, T., Abraham, I., Timmerman, W., et al. (2000). Beta-amyloid neurotoxicity is mediated by a glutamate-triggered excitotoxic cascade in rat nucleus basalis. European Journal of Neuroscience, 12, 2735–2745.
Harmeier, A., Wozny, C., Rost, B. R., et al. (2009). Role of amyloid-beta glycine 33 in oligomerization, toxicity, and neuronal plasticity. Journal of Neuroscience, 29, 7582–7590.
Harris, M. E., Carney, J. M., Cole, P. S., et al. (1995). Beta-amyloid peptide-derived, oxygen-dependent free radicals inhibit glutamate uptake in cultured astrocytes: Implications for Alzheimer’s disease. NeuroReport, 6, 1875–1879.
Hsieh, H., Boehm, J., Sato, C., et al. (2006). AMPAR removal underlies Abeta-induced synaptic depression and dendritic spine loss. Neuron, 52, 831–843.
Hu, M., Schurdak, M. E., Puttfarcken, P. S., El Kouhen, R., Gopalakrishnan, M., & Li, J. (2007). High content screen microscopy analysis of A beta 1-42-induced neurite outgrowth reduction in rat primary cortical neurons: Neuroprotective effects of alpha 7 neuronal nicotinic acetylcholine receptor ligands. Brain Research, 1151, 227–235.
Hu, N. W., Smith, I. M., Walsh, D. M., & Rowan, M. J. (2008). Soluble amyloid-beta peptides potently disrupt hippocampal synaptic plasticity in the absence of cerebrovascular dysfunction in vivo. Brain, 131, 2414–2424.
Hynd, M. R., Scott, H. L., & Dodd, P. R. (2004). Glutamate-mediated excitotoxicity and neurodegeneration in Alzheimer’s disease. Neurochemistry International, 45, 583–595.
Ishida, A., Furukawa, K., Keller, J. N., & Mattson, M. P. (1997). Secreted form of beta-amyloid precursor protein shifts the frequency dependency for induction of LTD, and enhances LTP in hippocampal slices. NeuroReport, 8, 2133–2137.
Janus, C., Pearson, J., McLaurin, J., et al. (2000). A beta peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer’s disease. Nature, 408, 979–982.
Ji, D., Lape, R., & Dani, J. A. (2001). Timing and location of nicotinic activity enhances or depresses hippocampal synaptic plasticity. Neuron, 31, 131–141.
Jolas, T., Zhang, X. S., Zhang, Q., et al. (2002). Long-term potentiation is increased in the CA1 area of the hippocampus of APP(swe/ind) CRND8 mice. Neurobiology of Diseases, 11, 394–409.
Jones, C. K., Brady, A. E., Davis, A. A., et al. (2008). Novel selective allosteric activator of the M1 muscarinic acetylcholine receptor regulates amyloid processing and produces antipsychotic-like activity in rats. Journal of Neuroscience, 28, 10422–10433.
Kabogo, D., Rauw, G., Amritraj, A., Baker, G., & Kar, S. (2008). Beta-amyloid-related peptides potentiate K(+)-evoked glutamate release from adult rat hippocampal slices. Neurobiology of Aging. doi:10.1016/j.neurobiolaging.2008.1008.1009.
Kamenetz, F., Tomita, T., Hsieh, H., et al. (2003). APP processing and synaptic function. Neuron, 37, 925–937.
Kang, J. E., Cirrito, J. R., Dong, H., Csernansky, J. G., & Holtzman, D. M. (2007). Acute stress increases interstitial fluid amyloid-beta via corticotropin-releasing factor and neuronal activity. Proceedings of the National Academy of Sciences of the United States of America, 104, 10673–10678.
Keller, J. N., Pang, Z., Geddes, J. W., et al. (1997). Impairment of glucose and glutamate transport and induction of mitochondrial oxidative stress and dysfunction in synaptosomes by amyloid beta-peptide: Role of the lipid peroxidation product 4-hydroxynonenal. Journal of Neurochemistry, 69, 273–284.
Kelly, B. L., & Ferreira, A. (2006). Beta-amyloid-induced dynamin 1 degradation is mediated by N-methyl-D-aspartate receptors in hippocampal neurons. Journal of Biological Chemistry, 281, 28079–28089.
Kenney, J. E., & Gould, T. W. (2008). Modulation of hippocampus-dependent learning and synaptic plasticity by nicotine. Molecular Neurobiology, 38, 101–121.
Kim, D., & Tsai, L. H. (2009). Bridging physiology and pathology in AD. Cell, 137, 997–1000.
Kim, J. H., Anwyl, R., Suh, Y. H., Djamgoz, M. B., & Rowan, M. J. (2001). Use-dependent effects of amyloidogenic fragments of (beta)-amyloid precursor protein on synaptic plasticity in rat hippocampus in vivo. Journal of Neuroscience, 21, 1327–1333.
Klyubin, I., Walsh, D. M., Cullen, W. K., et al. (2004). Soluble Arctic amyloid beta protein inhibits hippocampal long-term potentiation in vivo. European Journal of Neuroscience, 19, 2839–2846.
Klyubin, I., Betts, V., Welzel, A. T., et al. (2008). Amyloid beta protein dimer-containing human CSF disrupts synaptic plasticity: Prevention by systemic passive immunization. Journal of Neuroscience, 28, 4231–4237.
Klyubin, I., Wang, Q., Reed, M. N., et al. (2009). Protection against Aβ-mediated rapid disruption of synaptic plasticity and memory by memantine. Neurobiology of Aging. doi:10.1016/j.neurobiolaging.2009.04.005.
Koh, J. Y., Yang, L. L., & Cotman, C. W. (1990). Beta-amyloid protein increases the vulnerability of cultured cortical neurons to excitotoxic damage. Brain Research, 533, 315–320.
Kotermanski, S. E., & Johnson, J. W. (2009). Mg2+ imparts NMDA receptor subtype selectivity to the Alzheimer’s drug memantine. Journal of Neuroscience, 29, 2774–2779.
Kuchibhotla, K. V., Lattarulo, C. R., Hyman, B. T., & Bacskai, B. J. (2009). Synchronous hyperactivity and intercellular calcium waves in astrocytes in Alzheimer mice. Science, 323, 1211–1215.
Lacor, P. N., Buniel, M. C., Furlow, P. W., et al. (2007). Abeta oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer’s disease. Journal of Neuroscience, 27, 796–807.
Lambert, M. P., Barlow, A. K., Chromy, B. A., et al. (1998). Diffusible, nonfibrillar ligands derived from A beta(1-42) are potent central nervous system neurotoxins. Proceedings of the National Academy of Sciences of the United States of America, 95, 6448–6453.
Lau, C. G., & Zukin, R. S. (2007). NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nature Reviews Neuroscience, 8, 413–426.
Lauren, J., Gimbel, D. A., Nygaard, H. B., Gilbert, J. W., & Strittmatter, S. M. (2009). Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers. Nature, 457, 1128–1132.
Lawlor, B. A., & Davis, K. L. (1992). Does modulation of glutamatergic function represent a viable therapeutic strategy in Alzheimer’s disease? Biological Psychiatry, 31, 337–350.
Lee, D. H., & Wang, H. Y. (2003). Differential physiologic responses of alpha7 nicotinic acetylcholine receptors to beta-amyloid-40 and beta-amyloid1-42. Journal of Neurobiology, 55, 25–30.
Lesne, S., Koh, M. T., Kotilinek, L., et al. (2006). A specific amyloid-beta protein assembly in the brain impairs memory. Nature, 440, 352–357.
Léveillé, F., El Gaamouch, F., Gouix, E., et al. (2008). Neuronal viability is controlled by a functional relation between synaptic and extrasynaptic NMDA receptors. FASEB Journal, 22, 4258–4271.
Levin, E. D., Bradley, A., Addy, N., & Sigurani, N. (2002). Hippocampal alpha 7 and alpha 4 beta 2 nicotinic receptors and working memory. Neuroscience, 109, 757–765.
Li, S., Feig, L. A., & Hartley, D. M. (2007). A brief, but repeated, swimming protocol is sufficient to overcome amyloid beta-protein inhibition of hippocampal long-term potentiation. European Journal of Neuroscience, 26, 1289–1298.
Li, S., Hong, S., Shepardson, N. E., Walsh, D. M., Shankar, G. M., & Selkoe, D. (2009). Soluble oligomers of amyloid beta protein facilitate hippocampal long-term depression by disrupting neuronal glutamate uptake. Neuron, 62, 788–801.
Lipton, S. A. (2007). Pathologically activated therapeutics for neuroprotection. Nature Reviews Neuroscience, 8, 803–808.
Lorenzo, A., & Yankner, B. A. (1996). Amyloid fibril toxicity in Alzheimer’s disease and diabetes. Annals of the New York Academy of Sciences, 77, 89–95.
Lue, L. F., Kuo, Y. M., Roher, A. E., et al. (1999). Soluble amyloid beta peptide concentration as a predictor of synaptic change in Alzheimer’s disease. American Journal of Pathology, 155, 853–862.
Lynch, M. A. (2004). Long-term potentiation and memory. Physiological Reviews, 84, 87–136.
Ma, H., Lesne, S., Kotilinek, L., et al. (2007). Involvement of beta-site APP cleaving enzyme 1 (BACE1) in amyloid precursor protein-mediated enhancement of memory and activity-dependent synaptic plasticity. Proceedings of the National Academy of Sciences of the United States of America, 104, 8167–8172.
Machova, E., Jakubik, J., Michal, P., et al. (2008). Impairment of muscarinic transmission in transgenic APPswe/PS1dE9 mice. Neurobiology of Aging, 29, 368–378.
Mann, E. O., & Greenfield, S. A. (2003). Novel modulatory mechanisms revealed by the sustained application of nicotine in the guinea-pig hippocampus in vitro. Journal of Physiology, 551, 539–550.
Martin, S. E., de Fiebre, N. E., & de Fiebre, C. M. (2004). The alpha7 nicotinic acetylcholine receptor-selective antagonist, methyllycaconitine, partially protects against beta-amyloid1–42 toxicity in primary neuron-enriched cultures. Brain Research, 1022, 254–256.
Matos, M., Augusto, E., Oliveira, C. R., & Agostinho, P. (2008). Amyloid-beta peptide decreases glutamate uptake in cultured astrocytes: Involvement of oxidative stress and mitogen-activated protein kinase cascades. Neuroscience, 156, 898–910.
Matsuyama, S., Matsumoto, A., Enomoto, T., & Nishizaki, T. (2000). Activation of nicotinic acetylcholine receptors induces long-term potentiation in vivo in the intact mouse dentate gyrus. European Journal of Neuroscience, 12, 3741–3747.
Mattson, M. P., Cheng, B., Davis, D., Bryant, K., Lieberburg, I., & Rydel, R. E. (1992). Beta-amyloid peptides destabilize calcium homeostasis and render human cortical neurons vulnerable to excitotoxicity. Journal of Neuroscience, 12, 376–389.
Maurice, T., Lockhart, B. P., & Privat, A. (1996). Amnesia induced in mice by centrally administered beta-amyloid peptides involves cholinergic dysfunction. Brain Research, 706, 181–193.
McDonald, M. P., Dahl, E. E., Overmier, J. B., Mantyh, P., & Cleary, J. (1994). Effects of an exogenous beta-amyloid peptide on retention for spatial learning. Behavioral and Neural Biology, 62, 60–67.
McLean, C. A., Cherny, R. A., Fraser, F. W., et al. (1999). Soluble pool of A beta amyloid as a determinant of severity of neurodegeneration in Alzheimer’s disease. Annals of Neurology, 46, 860–866.
Molnar, Z., Soos, K., Lengyel, I., Penke, B., Szegedi, V., & Budai, D. (2004). Enhancement of NMDA responses by beta-amyloid peptides in the hippocampus in vivo. NeuroReport, 15, 1649–1652.
Morris, R. G., Moser, E. I., Riedel, G., et al. (2003). Elements of a neurobiological theory of the hippocampus: The role of activity-dependent synaptic plasticity in memory. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 358, 773–786.
Mousavi, M., & Hellstrom-Lindahl, E. (2009). Nicotinic receptor agonists and antagonists increase sAPPalpha secretion and decrease Abeta levels in vitro. Neurochemistry International, 54, 237–244.
Muller, U., Cristina, N., Li, Z. W., et al. (1994). Behavioral and anatomical deficits in mice homozygous for a modified beta-amyloid precursor protein gene. Cell, 79, 755–765.
Nakamura, S., Murayama, N., Noshita, T., Katsuragi, R., & Ohno, T. (2006). Cognitive dysfunction induced by sequential injection of amyloid-beta and ibotenate into the bilateral hippocampus; protection by memantine and MK-801. European Journal of Pharmacology, 548, 115–122.
Newhouse, P. A., Potter, A., & Singh, A. (2004). Effects of nicotinic stimulation on cognitive performance. Current Opinion in Pharmacology, 4, 36–46.
Nitta, A., Itoh, A., Hasegawa, T., & Nabeshima, T. (1994). Beta-amyloid protein-induced Alzheimer’s disease animal model. Neuroscience Letters, 170, 63–66.
Noda, M., Nakanishi, H., & Akaike, N. (1999). Glutamate release from microglia via glutamate transporter is enhanced by amyloid-beta peptide. Neuroscience, 92, 1465–1474.
Nordberg, A., Alafuzoff, I., & Winblad, B. (1992). Nicotinic and muscarinic subtypes in the human brain: Changes with aging and dementia. Journal of Neuroscience Research, 31, 103–111.
Oddo, S., & LaFerla, F. M. (2006). The role of nicotinic acetylcholine receptors in Alzheimer’s disease. Journal of Physiology (Paris), 99, 172–179.
Origlia, N., Righi, M., Capsoni, S., et al. (2008). Receptor for advanced glycation end product-dependent activation of p38 mitogen-activated protein kinase contributes to amyloid-beta-mediated cortical synaptic dysfunction. Journal of Neuroscience, 28, 3521–3530.
Parsons, C. G., Stoffler, A., & Danysz, W. (2007). Memantine: A NMDA receptor antagonist that improves memory by restoration of homeostasis in the glutamatergic system—too little activation is bad, too much is even worse. Neuropharmacology, 53, 699–723.
Pena, F., Gutierrez-Lerma, A., Quiroz-Baez, R., & Arias, C. (2006). The role of beta-amyloid protein in synaptic function: Implications for Alzheimer’s disease therapy. Current Neuropharmacology, 4, 149–163.
Pettit, D. L., Shao, Z., & Yakel, J. L. (2001). Beta-amyloid(1-42) peptide directly modulates nicotinic receptors in the rat hippocampal slice. Journal of Neuroscience, 21, RC120.
Phinney, A. L., Calhoun, M. E., Wolfer, D. P., Lipp, H. P., Zheng, H., & Jucker, M. (1999). No hippocampal neuron or synaptic bouton loss in learning-impaired aged beta-amyloid precursor protein-null mice. Neuroscience, 90, 1207–1216.
Plant, L. D., Boyle, J. P., Smith, I. F., Peers, C., & Pearson, H. A. (2003). The production of amyloid beta peptide is a critical requirement for the viability of central neurons. Journal of Neuroscience, 23, 5531–5535.
Puzzo, D., Privitera, L., Leznik, E., et al. (2008). Picomolar amyloid-beta positively modulates synaptic plasticity and memory in hippocampus. Journal of Neuroscience, 28, 14537–14545.
Ramsden, M., Plant, L. D., Webster, N. J., Vaughan, P. F., Henderson, Z., & Pearson, H. A. (2001). Differential effects of unaggregated and aggregated amyloid beta protein (1-40) on K(+) channel currents in primary cultures of rat cerebellar granule and cortical neurones. Journal of Neurochemistry, 79, 699–712.
Raymond, C. R., Ireland, D. R., & Abraham, W. C. (2003). NMDA receptor regulation by amyloid-beta does not account for its inhibition of LTP in rat hippocampus. Brain Research, 968, 263–272.
Reitz, C., Honig, L., Vonsattel, J. P., Tang, M. X., & Mayeux, R. (2009). Memory performance is related to amyloid and tau pathology in the hippocampus. Journal of Neurology, Neurosurgery and Psychiatry, 80, 715–721.
Ring, S., Weyer, S. W., Kilian, S. B., et al. (2007). The secreted beta-amyloid precursor protein ectodomain APPs alpha is sufficient to rescue the anatomical, behavioral, and electrophysiological abnormalities of APP-deficient mice. Journal of Neuroscience, 27, 7817–7826.
Roselli, F., Tirard, M., Lu, J., et al. (2005). Soluble beta-amyloid1-40 induces NMDA-dependent degradation of postsynaptic density-95 at glutamatergic synapses. Journal of Neuroscience, 25, 11061–11070.
Roth, M., Tomlinson, B. E., & Blessed, G. (1966). Correlation between scores for dementia and counts of ‘senile plaques’ in cerebral grey matter of elderly subjects. Nature, 209, 109–110.
Rowan, M. J., Klyubin, I., Cullen, W. K., & Anwyl, R. (2003). Synaptic plasticity in animal models of early Alzheimer’s disease. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 358, 821–828.
Rowan, M. J., Klyubin, I., Wang, Q., Hu, N. W., & Anwyl, R. (2007). Synaptic memory mechanisms: Alzheimer’s disease amyloid beta-peptide-induced dysfunction. Biochemical Society Transactions, 35, 1219–1223.
Santos-Torres, J., Fuente, A., Criado, J. M., Riolobos, A. S., Heredia, M., & Yajeya, J. (2007). Glutamatergic synaptic depression by synthetic amyloid beta-peptide in the medial septum. Journal of Neuroscience Research, 85, 634–648.
Savioz, A., Leuba, G., Vallet, P. G., & Walzer, C. (2009). Contribution of neural networks to Alzheimer disease’s progression. Brain Research Bulletin. doi:10.1016/j.brainresbull.2009.06.006.
Schliebs, R., & Arendt, T. (2006). The significance of the cholinergic system in the brain during aging and in Alzheimer’s disease. Journal of Neural Transmission, 113, 1625–1644.
Seabrook, G. R., Smith, D. W., Bowery, B. J., et al. (1999). Mechanisms contributing to the deficits in hippocampal synaptic plasticity in mice lacking amyloid precursor protein. Neuropharmacology, 38, 349–359.
Senechal, Y., Larmet, Y., & Dev, K. K. (2006). Unraveling in vivo functions of amyloid precursor protein: Insights from knockout and knockdown studies. Neurodegenerative Diseases, 3, 134–147.
Senechal, Y., Kelly, P. H., & Dev, K. K. (2008). Amyloid precursor protein knockout mice show age-dependent deficits in passive avoidance learning. Behavioural Brain Research, 186, 126–132.
Shankar, G. M., Bloodgood, B. L., Townsend, M., Walsh, D. M., Selkoe, D. J., & Sabatini, B. L. (2007). Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. Journal of Neuroscience, 27, 2866–2875.
Shankar, G. M., Li, S., Mehta, T. H., et al. (2008). Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nature Medicine, 14, 837–842.
Shinoe, T., Matsui, M., Taketo, M. M., & Manabe, T. (2005). Modulation of synaptic plasticity by physiological activation of M1 muscarinic acetylcholine receptors in the mouse hippocampus. Journal of Neuroscience, 25, 11194–11200.
Small, D. H. (2008). Network dysfunction in Alzheimer’s disease: Does synaptic scaling drive disease progression? Trends in Molecular Medicine, 14, 103–108.
Snyder, E. M., Nong, Y., Almeida, C. G., et al. (2005). Regulation of NMDA receptor trafficking by amyloid-beta. Nature Neuroscience, 8, 1051–1058.
Soriano, F. X., & Hardingham, G. E. (1997). Compartmentalized NMDA receptor signalling to survival and death. Journal of Physiology, 584, 381–387.
Srivareerat, M., Tran, T. T., Alzoubi, K. H., & Alkadhi, K. A. (2009a). Chronic psychosocial stress exacerbates impairment of cognition and long-term potentiation in beta-amyloid rat model of Alzheimer’s disease. Biological Psychiatry, 65, 918–926.
Srivareerat, M., Tran, T. T., Salim, S., Aleisa, A. M., & Alkadhi, K. A. (2009b). Chronic nicotine restores normal Abeta levels and prevents short-term memory and E-LTP impairment in Abeta rat model of Alzheimer’s disease. Neurobiology of Aging. doi:10.1016/j.neurobiolaging.2009.04.015.
Stephan, A., Laroche, S., & Davis, S. (2001). Generation of aggregated beta-amyloid in the rat hippocampus impairs synaptic transmission and plasticity and causes memory deficits. Journal of Neuroscience, 21, 5703–5714.
Szegedi, V., Juhasz, G., Budai, D., & Penke, B. (2005). Divergent effects of Abeta1-42 on ionotropic glutamate receptor-mediated responses in CA1 neurons in vivo. Brain Research, 1062, 120–126.
Taylor, C. J., Ireland, D. R., Ballagh, I., et al. (2008). Endogenous secreted amyloid precursor protein-alpha regulates hippocampal NMDA receptor function, long-term potentiation and spatial memory. Neurobiology of Diseases, 31, 250–260.
Teaktong, T., Graham, A. J., Court, J. A., et al. (2004). Nicotinic acetylcholine receptor immunohistochemistry in Alzheimer’s disease and dementia with Lewy bodies: Differential neuronal and astroglial pathology. Journal of the Neurological Sciences, 225, 39–49.
Terry, R. D. (1996). The pathogenesis of Alzheimer disease: An alternative to the amyloid hypothesis. Journal of Neuropathology and Experimental Neurology, 55, 1023–1025.
Tietje, K. R., Anderson, D. J., Bitner, R. S., et al. (2008). Preclinical characterization of A-582941: A novel alpha7 neuronal nicotinic receptor agonist with broad spectrum cognition-enhancing properties. CNS Neuroscience and Therapeutics, 14, 65–82.
Turner, P. R., O’Connor, K., Tate, W. P., & Abraham, W. C. (2003). Roles of amyloid precursor protein and its fragments in regulating neural activity, plasticity and memory. Progress in Neurobiology, 70, 1–32.
Verdier, Y., & Penke, B. (2004). Binding sites of amyloid beta-peptide in cell plasma membrane and implications for Alzheimer’s disease. Current Protein and Peptide Science, 5, 19–31.
Walsh, D. M., Klyubin, I., Fadeeva, J. V., et al. (2002). Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature, 416, 535–539.
Wang, J., Dickson, D. W., Trojanowski, J. Q., & Lee, V. M. (1999). The levels of soluble versus insoluble brain Abeta distinguish Alzheimer’s disease from normal and pathologic aging. Experimental Neurology, 158, 328–337.
Wang, H. Y., Lee, D. H., D’Andrea, M. R., Peterson, P. A., Shank, R. P., & Reitz, A. B. (2000). Beta-amyloid(1–42) binds to alpha7 nicotinic acetylcholine receptor with high affinity. Implications for Alzheimer’s disease pathology. Journal of Biological Chemistry, 275, 5626–5632.
Wang, Q., Walsh, D. M., Rowan, M. J., Selkoe, D. J., & Anwyl, R. (2004). Block of long-term potentiation by naturally secreted and synthetic amyloid beta-peptide in hippocampal slices is mediated via activation of the kinases c-Jun N-terminal kinase, cyclin-dependent kinase 5, and p38 mitogen-activated protein kinase as well as metabotropic glutamate receptor type 5. Journal of Neuroscience, 24, 3370–3378.
Wang, Q., Klyubin, I., Wright, S., Griswold-Prenner, I., Rowan, M. J., & Anwyl, R. (2007). Alpha v integrins mediate beta-amyloid induced inhibition of long-term potentiation. Neurobiology of Aging, 29, 1485–1493
Wang, H., Song, L., Laird, F., Wong, P. C., & Lee, H. K. (2008a). BACE1 knock-outs display deficits in activity-dependent potentiation of synaptic transmission at mossy fiber to CA3 synapses in the hippocampus. Journal of Neuroscience, 28, 8677–8681.
Wang, Q., Klyubin, I., Wright, S., Griswold-Prenner, I., Rowan, M. J., & Anwyl, R. (2008b). Alpha v integrins mediate beta-amyloid induced inhibition of long-term potentiation. Neurobiology of Aging, 29, 1485–1493.
Warpman, U., Alafuzoff, I., & Nordberg, A. (1993). Coupling of muscarinic receptors to GTP proteins in postmortem human brain—alterations in Alzheimer’s disease. Neuroscience Letters, 150, 39–43.
Wasling, P., Daborg, J., Riebe, I., et al. (2009). Synaptic retrogenesis and amyloid-beta in Alzheimer’s disease. Journal of Alzheimers Disease, 16, 1–14.
Welsby, P., Rowan, M., & Anwyl, R. (2006). Nicotinic receptor-mediated enhancement of long-term potentiation involves activation of metabotropic glutamate receptors and ryanodine-sensitive calcium stores in the dentate gyrus. European Journal of Neuroscience, 24, 3109–3118.
Welsby, P. J., Rowan, M. J., & Anwyl, R. (2007). Beta-amyloid blocks high frequency stimulation induced LTP but not nicotine enhanced LTP. Neuropharmacology, 53, 188–195.
Welsby, P. J., Rowan, M. J., & Anwyl, R. (2009). Intracellular mechanisms underlying the nicotinic enhancement of LTP in the rat dentate gyrus. European Journal of Neuroscience, 29, 65–75.
Wevers, A., Monteggia, L., Nowacki, S., et al. (1999). Expression of nicotinic acetylcholine receptor subunits in the cerebral cortex in Alzheimer’s disease: Histotopographical correlation with amyloid plaques and hyperphosphorylated-tau protein. European Journal of Neuroscience, 11, 2551–2565.
Wevers, A., Burghaus, L., Moser, N., et al. (2000). Expression of nicotinic acetylcholine receptors in Alzheimer’s disease: Postmortem investigations and experimental approaches. Behavioural Brain Research, 113, 207–215.
Woolf, N. J. (1991). Cholinergic systems in mammalian brain and spinal cord. Progress in Neurobiology, 37, 475–524.
Wright, S., Malinin, N. L., Powell, K. A., Yednock, T., Rydel, R. E., & Griswold-Prenner, I. (2007). Alpha2beta1 and alphaVbeta1 integrin signaling pathways mediate amyloid-beta-induced neurotoxicity. Neurobiology of Aging, 28, 226–237.
Wrighton, D. C., Baker, E. J., Chen, P. E., & Wyllie, D. J. A. (2008). Mg2+ and memantine block of rat recombinant NMDA receptors containing chimeric NR2A/2D subunits expressed in Xenopus laevis oocytes. Journal of Physiology, 586, 211–225.
Wu, J., Anwyl, R., & Rowan, M. J. (1995a). Beta-amyloid selectively augments NMDA receptor-mediated synaptic transmission in rat hippocampus. NeuroReport, 6, 2409–2413.
Wu, J., Anwyl, R., & Rowan, M. J. (1995b). Beta-amyloid-(1-40) increases long-term potentiation in rat hippocampus in vitro. European Journal of Pharmacology, 284, R1–R3.
Wu, M. N., He, Y. X., Guo, F., & Qi, J. S. (2008). Alpha4beta2 nicotinic acetylcholine receptors are required for the amyloid beta protein-induced suppression of long-term potentiation in rat hippocampal CA1 region in vivo. Brain Research Bulletin, 77, 84–90.
Yamazaki, Y., Hamaue, N., & Sumikawa, K. (2002). Nicotine compensates for the loss of cholinergic function to enhance long-term potentiation induction. Brain Research, 946, 148–152.
Yan, S. D., Bierhaus, A., Nawroth, P. P., & Stern, D. M. (2009). RAGE and Alzheimer’s disease: A progression factor for amyloid-beta-induced cellular perturbation? Journal of Alzheimers Disease, 16, 833–843.
Yang, T., Knowles, J. K., Lu, Q., et al. (2008). Small molecule, non-peptide p75 ligands inhibit Abeta-induced neurodegeneration and synaptic impairment. PLoS ONE, 3, e3604.
Ye, C., Walsh, D. M., Selkoe, D. J., & Hartley, D. M. (2004). Amyloid beta-protein induced electrophysiological changes are dependent on aggregation state: N-methyl-D-aspartate (NMDA) versus non-NMDA receptor/channel activation. Neuroscience Letters, 366, 320–325.
Zajaczkowski, W., Frankiewicz, T., Parsons, C. G., & Danysz, W. (1997). Uncompetitive NMDA receptor antagonists attenuate NMDA-induced impairment of passive avoidance learning and LTP. Neuropharmacology, 36, 961–971.
Zhang, S. J., Steijaert, M. N., Lau, D., et al. (2007). Decoding NMDA receptor signaling: Identification of genomic programs specifying neuronal survival and death. Neuron, 53, 549–562.
Zheng, H., Jiang, M., Trumbauer, M. E., et al. (1995). Beta-amyloid precursor protein-deficient mice show reactive gliosis and decreased locomotor activity. Cell, 81, 525–531.
Zorumski, C. F., & Izumi, Y. (1998). Modulation of LTP induction by NMDA receptor activation and nitric oxide release. Progress in Brain Research, 118, 173–182.
Acknowledgments
The authors wish to acknowledge the support of Science Foundation Ireland, the Health Research Board of Ireland, IRCSET, GSK and the Irish Development Authority.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ondrejcak, T., Klyubin, I., Hu, NW. et al. Alzheimer’s Disease Amyloid β-Protein and Synaptic Function. Neuromol Med 12, 13–26 (2010). https://doi.org/10.1007/s12017-009-8091-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12017-009-8091-0