Abstract
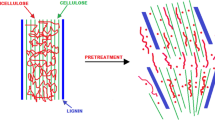
Biomass alkaline deacetylation prior to acid pretreatment can be a promising alternative to reduce the toxicity of hemicellulosic hydrolysates and improve second-generation bioethanol production. In this paper, the effect of alkaline deacetylation of sugarcane bagasse on bioethanol production by Spathaspora passalidarum was evaluated. Sugarcane bagasse deacetylated hemicellulosic hydrolysate (DHH) was processed using the following sequence: (1) deacetylation (0.4%, w/v NaOH, 70 °C, 3 h) and (2) acid pretreatment (0.5% (v/v) of H2SO4, 140 °C, 15 min). Non-deacetylated, hemicellulosic hydrolysate (HH), was obtained applying only acid pretreatment (0.5% (v/v) of H2SO4, 140 °C, 15 min). Biomass deacetylation reduced the content of acetic acid and some phenolic compounds in the hydrolysate (DHH) compared to acid pretreatment (HH), which resulted in its low toxicity. Thus, the bioethanol production with DHH was of 16.92 g L−1, whereas only 1.3 g L−1 of bioethanol was obtained with HH fermentation. Deacetylation process provided a 13-fold increase in bioethanol production by S. passalidarum, showing that alkaline deacetylation followed by sulfuric acid pretreatment is a promising strategy to increase bioethanol production. This procedure provided a simple and practical alternative to the classic methods of detoxification of hemicellulosic hydrolysate from sugarcane bagasse.




Similar content being viewed by others
References
Gupta A, Verma JP (2015) Sustainable bio-ethanol production from agro-residues: a review. Renew Sust Energ Rev 41:550–567. https://doi.org/10.1016/j.rser.2014.08.032
Zabed H, Sahu JN, Boyce AN, Faruq G (2016) Fuel ethanol production from lignocellulosic biomass: an overview on feedstocks and technological approaches. Renew Sust Energ Rev 66:751–774. https://doi.org/10.1016/j.rser.2016.08.038
Thornley P, Gilbert P, Shackley S, Hammond J (2015) Maximizing the greenhouse gas reductions from biomass: the role of life cycle assessment. Biomass Bioenergy 81:35–43. https://doi.org/10.1016/j.biombioe.2015.05.002
Mandegari MA, Farzad S, Görgens JF (2017) Recent trends on techno-economic assessment (TEA) of sugarcane biorefineries. Biofuel Res J 4:704–712. https://doi.org/10.18331/BRJ2017.4.3.7
Szczerbowski D, Pitarelo AP, Zandoná Filho A, Ramos LP (2014) Sugarcane biomass for biorefineries: comparative composition of carbohydrate and non-carbohydrate components of bagasse and straw. Carbohydr Polym 114:95–101. https://doi.org/10.1016/j.carbpol.2014.07.052
Zabed H, Sahu JN, Suely A et al (2017) Bioethanol production from renewable sources: current perspectives and technological progress. Renew Sust Energ Rev 71:475–501. https://doi.org/10.1016/j.rser.2016.12.076
Moysés DN, Reis VCB, Almeida JRM et al (2016) Xylose fermentation by Saccharomyces cerevisiae: challenges and prospects. Int J Mol Sci 17:1–18. https://doi.org/10.3390/ijms17030207
Hou J, Qiu C, Shen Y et al (2017) Engineering of Saccharomyces cerevisiae for the efficient co-utilization of glucose and xylose. FEMS Yeast Res 17. https://doi.org/10.1093/femsyr/fox034
Bonan CIDG, Biazi LE, Dionísio SR et al (2020) Redox potential as a key parameter for monitoring and optimization of xylose fermentation with yeast Spathaspora passalidarum under limited-oxygen conditions. Bioprocess Biosyst Eng 43:1509–1519. https://doi.org/10.1007/s00449-020-02344-2
Cadete RM, Las Heras AM, Sandström AG et al (2016) Exploring xylose metabolism in Spathaspora species: XYL1.2 from Spathaspora passalidarum as the key for efficient anaerobic xylose fermentation in metabolic engineered Saccharomyces cerevisiae. Biotechnol Biofuels 9:1–14. https://doi.org/10.1186/s13068-016-0570-6
Su YK, Willis LB, Jeffries TW (2015) Effects of aeration on growth, ethanol and polyol accumulation by Spathaspora passalidarum NRRL Y-27907 and Scheffersomyces stipitis NRRL Y-7124. Biotechnol Bioeng 112:457–469. https://doi.org/10.1002/bit.25445
Long TM, Su Y-K, Headman J et al (2012) Cofermentation of glucose, xylose, and cellobiose by the beetle-associated yeast Spathaspora passalidarum. Appl Environ Microbiol 78:5492–5500. https://doi.org/10.1128/AEM.00374-12
Nakanishi SC, Soares LB, Biazi LE et al (2017) Fermentation strategy for second generation ethanol production from sugarcane bagasse hydrolyzate by Spathaspora passalidarum and Scheffersomyces stipitis. Biotechnol Bioeng 114:2211–2221. https://doi.org/10.1002/bit.26357
Soares LB, Bonan CIDG, Biazi LE et al (2020) Investigation of hemicellulosic hydrolysate inhibitor resistance and fermentation strategies to overcome inhibition in non-saccharomyces species. Biomass Bioenergy 137:105549. https://doi.org/10.1016/j.biombioe.2020.105549
Yu H, Guo J, Chen Y et al (2017) Efficient utilization of hemicellulose and cellulose in alkali liquor-pretreated corncob for bioethanol production at high solid loading by Spathaspora passalidarum U1–58. Bioresour Technol 232:168–175. https://doi.org/10.1016/j.biortech.2017.01.077
Neitzel T, Lima CS, Biazi LE et al (2020) Impact of the Melle-Boinot process on the enhancement of second-generation ethanol production by Spathaspora passalidarum. Renew Energy 160:1206–1216. https://doi.org/10.1016/j.renene.2020.07.027
Hou X, Yao S (2012) Improved inhibitor tolerance in xylose-fermenting yeast Spathaspora passalidarum by mutagenesis and protoplast fusion. Appl Microbiol Biotechnol 93:2591–2601. https://doi.org/10.1007/s00253-011-3693-5
Van der Pol EC, Bakker RR, Baets P, Eggink G (2014) By-products resulting from lignocellulose pretreatment and their inhibitory effect on fermentations for (bio)chemicals and fuels. Appl Microbiol Biotechnol 98:9579–9593. https://doi.org/10.1007/s00253-014-6158-9
Jönsson LJ, Martín C (2016) Pretreatment of lignocellulose: formation of inhibitory by-products and strategies for minimizing their effects. Bioresour Technol 199:103–112. https://doi.org/10.1016/j.biortech.2015.10.009
Roque LR, Morgado GP, Nascimento VM et al (2019) Liquid-liquid extraction: a promising alternative for inhibitors removing of pentoses fermentation. Fuel 242:775–787. https://doi.org/10.1016/j.fuel.2018.12.130
Canilha L, Santos VTO, Rocha GJM et al (2011) A study on the pretreatment of a sugarcane bagasse sample with dilute sulfuric acid. J Ind Microbiol Biotechnol 38:1467–1475. https://doi.org/10.1007/s10295-010-0931-2
Rocha GJM, Gonçalves AR, Nakanishi SC et al (2015) Pilot scale steam explosion and diluted sulfuric acid pretreatments: Comparative study aiming the sugarcane bagasse saccharification. Ind Crop Prod 74:810–816. https://doi.org/10.1016/j.indcrop.2015.05.074
Jönsson LJ, Alriksson B, Nilvebrant N-O (2013) Bioconversion of lignocellulose: inhibitors and detoxification. Biotechnol Biofuels 6:1–10. https://doi.org/10.1186/1754-6834-6-16
de Castro RC, A, Fonseca BG, dos Santos HTL et al (2017) Alkaline deacetylation as a strategy to improve sugars recovery and ethanol production from rice straw hemicellulose and cellulose. Ind Crop Prod 106:65–73. https://doi.org/10.1016/j.indcrop.2016.08.053
Kundu C, Lee HJ, Lee JW (2015) Enhanced bioethanol production from yellow poplar by deacetylation and oxalic acid pretreatment without detoxification. Bioresour Technol 178:28–35. https://doi.org/10.1016/j.biortech.2014.08.082
Salvachúa D, Mohagheghi A, Smith H et al (2016) Succinic acid production on xylose-enriched biorefinery streams by Actinobacillus succinogenes in batch fermentation. Biotechnol Biofuels 9:1–15. https://doi.org/10.1186/s13068-016-0425-1
Salvachúa D, Smith H, St. John PC et al (2016) Succinic acid production from lignocellulosic hydrolysate by Basfia succiniciproducens. Bioresour Technol 214:558–566. https://doi.org/10.1016/j.biortech.2016.05.018
Morales P, Gentina JC, Aroca G, Mussatto SI (2017) Development of an acetic acid tolerant Spathaspora passalidarum strain through evolutionary engineering with resistance to inhibitors compounds of autohydrolysate of Eucalyptus globulus. Ind Crop Prod 106:5–11. https://doi.org/10.1016/j.indcrop.2016.12.023
Lima CS, Rabelo SC, Ciesielski PN et al (2018) Multiscale alterations in sugar cane cagasse and straw submitted to alkaline deacetylation. ACS Sustain Chem Eng 6:3796–3804. https://doi.org/10.1021/acssuschemeng.7b04158
Fonseca BG, Mateo S, Roberto IC et al (2020) Bioconversion in batch bioreactor of olive-tree pruning biomass optimizing treatments for ethanol production. Biochem Eng J 164:107793. https://doi.org/10.1016/j.bej.2020.107793
Mateo S, Mateo P, Barbanera M et al (2020) Acid hydrolysis of olive tree leaves: preliminary study towards biochemical conversion. Processes 8:1–13. https://doi.org/10.3390/pr8080886
Peinado S, Mateo S, Sánchez S, Moya AJ (2019) Effectiveness of sodium borohydride treatment on acid hydrolyzates from olive-tree pruning biomass for bioethanol production. BioEnergy Res 12:302–311. https://doi.org/10.1007/s12155-019-09979-4
Santoro DCJ, Assis T, Dionisio SR, et al (2015) Scaling up dilute sulfuric acid pretreatment for sugarcane bagasse bioethanol production. 37th Symp. Biotechnol. Fuels Chem
Santos SC, Sousa AS, Dionísio SR et al (2016) Bioethanol production by recycled Scheffersomyces stipitis in sequential batch fermentations with high cell density using xylose and glucose mixture. Bioresour Technol 219:319–329. https://doi.org/10.1016/j.biortech.2016.07.102
Sluiter JB, Chum H, Gomes AC et al (2016) Evaluation of Brazilian sugarcane bagasse characterization: an interlaboratory comparison study. J AOAC Int 99:579–585. https://doi.org/10.5740/jaoacint.15-0063
Mok WSL, Antal MJ (1992) Uncatalyzed solvolysis of whole biomass hemicellulose by hot compressed liquid water. Ind Eng Chem Res 31:1157–1161. https://doi.org/10.1021/ie00004a026
Gouveia ER, do Nascimento RT, Souto-Maior AM, de Rocha GJ, M (2009) Validação de metodologia para a caracterização química de bagaço de cana-de-açúcar. Quim Nova 32:1500–1503. https://doi.org/10.1590/S0100-40422009000600026
Gilliland RB (1959) Determination of yeast viability. J Inst Brew 65:424–429. https://doi.org/10.1002/j.2050-0416.1959.tb01482.x
Wang W, Chen X, Katahira R, Tucker M (2019) Characterization and deconstruction of oligosaccharides in black liquor from deacetylation process of corn stover. Front Energy Res 7:1–10. https://doi.org/10.3389/fenrg.2019.00054
Kohli K, Prajapati R, Sharma B (2019) Bio-based chemicals from renewable biomass for integrated biorefineries. Energies 12:1–40. https://doi.org/10.3390/en12020233
Decostanzi M, Auvergne R, Boutevin B, Caillol S (2019) Biobased phenol and furan derivative coupling for the synthesis of functional monomers. Green Chem 21:724–747. https://doi.org/10.1039/C8GC03541E
Chen X, Wang W, Ciesielski P et al (2016) Improving sugar yields and reducing enzyme loadings in the deacetylation and mechanical refining (DMR) process through multistage disk and szego refining and corresponding techno-economic analysis. ACS Sustain Chem Eng 4:324–333. https://doi.org/10.1021/acssuschemeng.5b01242
Chen X, Shekiro J, Elander R, Tucker M (2012) Improved xylan hydrolysis of corn stover by deacetylation with high solids dilute acid pretreatment. Ind Eng Chem Res 51:70–76. https://doi.org/10.1021/ie201493g
Shekiro J III, Kuhn EM, Nagle NJ et al (2014) Characterization of pilot-scale dilute acid pretreatment performance using deacetylated corn stover. Biotechnol Biofuels 7:1–10. https://doi.org/10.1186/1754-6834-7-23
Cheng K-K, Cai B-Y, Zhang J-A et al (2008) Sugarcane bagasse hemicellulose hydrolysate for ethanol production by acid recovery process. Biochem Eng J 38:105–109. https://doi.org/10.1016/j.bej.2007.07.012
Senatham S, Chamduang T, Kaewchingduang Y et al (2016) Enhanced xylose fermentation and hydrolysate inhibitor tolerance of Scheffersomyces shehatae for efficient ethanol production from non-detoxified lignocellulosic hydrolysate. Springerplus 5:1–8. https://doi.org/10.1186/s40064-016-2713-4
Canilha L, Carvalho W, Felipe MGA et al (2010) Ethanol production from sugarcane bagasse hydrolysate using Pichia stipitis. Appl Biochem Biotechnol 161:84–92. https://doi.org/10.1007/s12010-009-8792-8
Xu F, Sun RC, Sun JX et al (2005) Determination of cell wall ferulic and p-coumaric acids in sugarcane bagasse. Anal Chim Acta 552:207–217. https://doi.org/10.1016/j.aca.2005.07.037
Ou SY, Luo YL, Huang CH, Jackson M (2009) Production of coumaric acid from sugarcane bagasse. Innov Food Sci Emerg Technol 10:253–259. https://doi.org/10.1016/j.ifset.2008.10.008
Chandel AK, Kapoor RK, Singh A, Kuhad RC (2007) Detoxification of sugarcane bagasse hydrolysate improves ethanol production by Candida shehatae NCIM 3501. Bioresour Technol 98:1947–1950. https://doi.org/10.1016/j.biortech.2006.07.047
Toquero C, Bolado S (2014) Effect of four pretreatments on enzymatic hydrolysis and ethanol fermentation of wheat straw. Influence of inhibitors and washing. Bioresour Technol 157:68–76. https://doi.org/10.1016/j.biortech.2014.01.090
Wang X, Jin M, Balan V et al (2014) Comparative metabolic profiling revealed limitations in xylose-fermenting yeast during co-fermentation of glucose and xylose in the presence of inhibitors. Biotechnol Bioeng 111:152–164. https://doi.org/10.1002/bit.24992
Guo Z, Olsson L (2014) Physiological response of Saccharomyces cerevisiae to weak acids present in lignocellulosic hydrolysate. FEMS Yeast Res 14:1234–1248. https://doi.org/10.1111/1567-1364.12221
Fitzgerald DJ, Stratford M, Gasson MJ et al (2004) Mode of antimicrobial action of vanillin against Escherichia coli, Lactobacillus plantarum and Listeria innocua. J Appl Microbiol 97:104–113. https://doi.org/10.1111/j.1365-2672.2004.02275.x
Liu ZL (2011) Molecular mechanisms of yeast tolerance and in situ detoxification of lignocellulose hydrolysates. Appl Microbiol Biotechnol 90:809–825. https://doi.org/10.1007/s00253-011-3167-9
Adeboye PT, Bettiga M, Olsson L (2017) ALD5, PAD1, ATF1 and ATF2 facilitate the catabolism of coniferyl aldehyde, ferulic acid and p-coumaric acid in Saccharomyces cerevisiae. Sci Rep 7:1–13. https://doi.org/10.1038/srep42635
Martiniano SE, Chandel AK, Soares LCSR et al (2013) Evaluation of novel xylose-fermenting yeast strains from Brazilian forests for hemicellulosic ethanol production from sugarcane bagasse. 3. Biotech 3:345–352. https://doi.org/10.1007/s13205-013-0145-1
Dussán KJ, Silva DDV, Perez VH, da Silva SS (2016) Evaluation of oxygen availability on ethanol production from sugarcane bagasse hydrolysate in a batch bioreactor using two strains of xylose-fermenting yeast. Renew Energy 87:703–710. https://doi.org/10.1016/j.renene.2015.10.065
Ferreira AD, Mussatto SI, Cadete RM et al (2011) Ethanol production by a new pentose-fermenting yeast strain, Scheffersomyces stipitis UFMG-IMH 43.2, isolated from the Brazilian forest. Yeast 28:547–554. https://doi.org/10.1002/yea.1858
Canilha L, Chandel AK, Suzane dos Santos Milessi T, et al (2012) Bioconversion of sugarcane biomass into ethanol: an overview about composition, pretreatment methods, detoxification of hydrolysates, enzymatic saccharification, and ethanol fermentation. J Biomed Biotechnol 1:1–15. https://doi.org/10.1155/2012/989572
Mateo S, Roberto IC, Sánchez S, Moya AJ (2013) Detoxification of hemicellulosic hydrolyzate from olive tree pruning residue. Ind Crop Prod 49:196–203. https://doi.org/10.1016/j.indcrop.2013.04.046
Martien JI, Amador-Noguez D (2017) Recent applications of metabolomics to advance microbial biofuel production. Curr Opin Biotechnol 43:118–126. https://doi.org/10.1016/j.copbio.2016.11.006
Narayanan V, Sànchez i Nogué V, van Niel EWJ, Gorwa-Grauslund MF (2016) Adaptation to low pH and lignocellulosic inhibitors resulting in ethanolic fermentation and growth of Saccharomyces cerevisiae. AMB Express 6:1–13. https://doi.org/10.1186/s13568-016-0234-8
Nielsen F, Tomás-Pejó E, Olsson L, Wallberg O (2015) Short-term adaptation during propagation improves the performance of xylose-fermenting Saccharomyces cerevisiae in simultaneous saccharification and co-fermentation. Biotechnol Biofuels 8:1–15. https://doi.org/10.1186/s13068-015-0399-4
Cadete RM, Melo MA, Dussán KJ et al (2012) Diversity and physiological characterization of D-xylose-fermenting yeasts isolated from the Brazilian Amazonian Forest. PLoS One 7:1–11. https://doi.org/10.1371/journal.pone.0043135
Funding
This study was financed in part by the Brazilian funding agencies Coordination for the Improvement of Higher Education Personnel (CAPES – Finance code 001), the National Council for Scientific and Technological Development (CNPq, grant no. 455549/2014-1), and the Brazilian Biorenewables National Laboratory - LNBR (CNPEM/MCTIC). The latter provided open access to the Bioprocesses (BPC) facility.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lima, C.S., Neitzel, T., de Oliveira Pereira, I. et al. Effect of the Sugarcane Bagasse Deacetylation in the Pentoses Fermentation Process. Bioenerg. Res. 14, 1171–1183 (2021). https://doi.org/10.1007/s12155-020-10243-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-020-10243-3