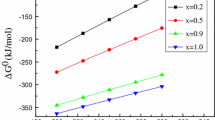
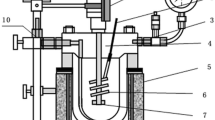
Abstract
Red mud produced in the Bayer process is a hazardous solid waste because of its high alkalinity; however, it is rich in valuable components such as titanium, iron, and aluminum. In this study, a novel calcification–carbonation method was developed to recover alkali and alumina from Bayer red mud under mild reaction conditions. Batch experiments were performed to evaluate the potential effects of important parameters such as temperature, amount of CaO added, and CO2 partial pressure on the recovery of alkali and alumina. The results showed that 95.2% alkali and 75.0% alumina were recovered from red mud with decreases in the mass ratios of Na2O to Fe2O3 and of Al2O3 to Fe2O3 from 0.42 and 0.89 to 0.02 and 0.22, respectively. The processed red mud with less than 0.5wt% Na2O can potentially be used as a construction material.
Similar content being viewed by others
References
H.L. Chen and Q. Long, Reactive characteristics and mechanism analysis of the removal of sodium and potassium from the Bayer red mud by calcium oxide with ultrasound assistance, Saf. Environ. Eng., 22(2015), No. 3, p. 40.
S. Agatzini-Leonardou, P. Oustadakis, P.E. Tsakiridis, and Ch. Markopoulos, Titanium leaching from red mud by diluted sulfuric acid at atmospheric pressure, J. Hazard. Mater., 157(2008), No. 2-3, p. 579.
X.L. Nan, T.A. Zhang, Y. Liu, and Z.H. Dou, Analysis of comprehensive utilization of red mud in China, Chin. J. Process. Eng., 10(2010), No. S1, p. 264.
G. Power, M. Gräfe, and C. Klauber, Bauxite residue issues: I. Current management, disposal and storage practices, Hydrometallurgy., 108(2011), No. 1-2, p. 33.
W.C. Liu, Study on the Multiphase Transformation of Bayer Red Mud in the High Temperature Roasting Reaction and Recovery of Iron Aluminum and Sodium [Dissertation], Huazhong University of Science and Technology, Wuhan, 2010, p. 9.
C. Klauber, M. Gräfe, and G. Power, Bauxite residue issues: II. options for residue utilization, Hydrometallurgy, 108(2011), No. 1-2, p. 11.
P.E. Tsakiridis, S. Agatzini-Leonardou, and P. Oustadakis, Red mud addition in the raw meal for the production of Portland cement clinker, J. Hazard. Mater., 116(2004), No. 1-2, p. 103.
S. Qin and B.L. Wu, Effect of self-glazing on reducing the radioactivity levels of red mud based ceramic materials, J. Hazard. Mater., 198(2011), p. 269.
A. Tor, N. Danaoglu, G. Arslan, and Y. Cengeloglu, Removal of fluoride from water by using granular red mud: Batch and column studies, J. Hazard. Mater., 164(2009), No. 1, p. 271.
C.W. Gray, S.J. Dunham, P.G. Dennis, F.J. Zhao, and S.P. McGrath, Field evaluation of in situ remediation of a heavy metal contaminated soil using lime and red-mud, Enviton. Pollut., 142(2006), No. 3, p. 530.
W.C. Liu, S.Y. Sun, L. Zhang, S. Jahanshahi, and J.K. Yang, Experimental and simulative study on phase transformation in Bayer red mud soda-lime roasting system and recovery of Al, Na and Fe, Miner. Eng., 39(2012), p. 213.
X.B. Li, W. Xiao, W. Liu, G.H. Liu, Z.H. Peng, Q.S. Zhou, and T.G. Qi, Recovery of alumina and ferric oxide from Bayer red mud rich in iron by reduction sintering, Trans. Nonferous Met. Soc. China, 19(2009), No. 5, p. 1342.
L. Zhong, Y.F. Zhang, and Y. Zhang, Extraction of alumina and sodium oxide from red mud by a mild hydro-chemical process, J. Hazard. Mater., 172(2009), No. 2-3, p. 1629.
X.L. Pan, H.Y. Yu, and G.F. Tu, Reduction of alkalinity in bauxite residue during Bayer digestion in high-ferrite diasporic bauxite, Hydrometallurgy, 151(2015), p. 98.
G.Z. Lu, T.A. Zhang, X.F. Zhu, Y. Liu, Y.X. Wang, F.F. Guo, Q.Y. Zhao, and C.Z. Zheng, Calcification-carbonation method for cleaner alumina production and CO2 utilization, JOM, 66(2014) No. 9, p. 1616.
M. Authier-Martin, G. Forte, S. Ostap, and J. See, The mineralogy of bauxite for producing smelter-grade alumina, JOM, 53(2001), No. 12, p. 36.
J.Y. Ma, K.M. Zhai, and Z.B. Li, Desilication of synthetic Bayer liquor with calcium sulfate dihydrate: kinetics and modelling, Hydrometallurgy, 107(2011), No. 1-2, p. 48.
S. Peter, The processing of high silica bauxites-review of existing and potential processes, Hydrometallurgy, 98(2009), No. 1-2, p. 162.
G. Baksa, F. Vallo, F. Sitkei, J. Zoldi, and K. Solymar, Complex causticisation: an effective means for the reduction of NaOH losses in an alumina plant, [in] TMS 1986 Annual Meeting and Exhibition, Warrendale, 1986, p. 75.
R. Zhang, S.L. Zheng, S.H Ma, and Y. Zhang, Recovery of alumina and alkali in Bayer red mud by the formation of andradite- grossular hydrogarnet in hydrothermal process, J. Hazard. Mater., 189(2011), No. 3, p. 827.
T.A. Zhang, G.Z. Lv, Y. Liu, Z.H. Dou, Q.Y. Zhao, L.P. Niu and J.C. He, A Method of consumption Bayer Red Mud, Chinese Patent, Appl. 10275030.X, 2011.
J. Mon, Y. Deng, M. Flury, and J.B. Harsh, Cesium incorporation and diffusion in cancrinite, sodalite, zeolite, and allophone, Microporous Mesoporous Mater., 86(2005), No. 1-3, p. 277.
N.J. Kenyon and M.T. Weller, The effect of calcium on phase formation in the sodium aluminium silicate carbonate system and the structure of NaCaSiO3OH, Microporous Mesoporous Mater., 59(2003), No. 2-3, p. 185.
J.M. Rivas Mercury, P. Pena, A.H. De Aza, X. Turrillas, I. Sobrados, and J. Sanz, Solid-state 27Al and 29Si NMR investigations on Si-substituted hydrogarnets, Acta Mater., 55(2007), No. 4, p. 1183.
S.W. You, Y.F. Zhang, F.F. Chen, S.T. Cao, and Y. Zhang, Transformation of NaCaHSiO4 to sodalite and katoite in sodium aluminate solution, Hydrometallurgy, 141(2014), p. 43.
K. Kawai and T. Tsuchiya, Elasticity and phase stability of pyrope garnet from ab initio computation, Phys. Earth Planet. Inter., 240(2015), p. 125.
H.L. Li and S.Q. Gu, Technology Handbook of Aluminum Production, 1st Vol., Metallutgical Industry Press., Beijing, 2011, p. 415.
H.B. Zhang, Theoretical Study on the Carbon Dioxide Neutralization Treatment of Hydrated Calcium Aluminates [Dissertation], Central South University, Changsha, 2010, p. 34.
Y. Liu, C.X. Lin, and Y.G. Wu, Characterization of red mud derived from a combined Bayer process and bauxite calcination method, J. Hazard. Mater., 146(2007), No. 1-2, p. 255.
C.A. Ríos, C.D. Williams, and M.A. Fullen, Hydrothermal synthesis of hydrogarnet and tobermorite at 175°C from kaolinite and metakaolinite in the CaO-Al2O3-SiO2-H2O system: a comparative study, Appl. Clay Sci., 43(2009), No. 2, p. 228.
Y.S. He, Z. Li, H.X. Xi, J.G. Guo, and Q.B. Xia, Research progress of gas-solid adsorption isotherms, Ion Exch. Adsorpt., 20(2004), No. 4, p. 376.
X.J. Liu, J. Xiong, and L.X. Liang, Investigation of pore structure and fractal characteristics of organic-rich Yanchang formation shale in central China by nitrogen adsorption/desorption analysis, J. Nat. Gas Sci. Eng., 22(2015), p. 62.
G.A. Tompsett, L. Krogh, D.W. Griffin, and W.C. Conner, Hysteresis and scanning behavior of mesoporous molecular sieves, Langmuir, 21(2005), No. 18, p. 8214.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhu, Xf., Zhang, Ta., Wang, Yx. et al. Recovery of alkali and alumina from Bayer red mud by the calcification–carbonation method. Int J Miner Metall Mater 23, 257–268 (2016). https://doi.org/10.1007/s12613-016-1234-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12613-016-1234-z