Abstract
Production of liquid biofuels derived from vegetable oils in recent years significantly increased which causes surplus of by-product (waste glycerol) from this process. Therefore it is of great importance to find cheap and fast method of use its utilization. In this study, a possibility of the utilization of technical purity glycerin as an addition to wood pellets intended for heating purposes has been investigated. Usefulness of pellets contained glycerol additions has been compared in terms of applicable quality standards for wood pellets. Effects of waste glycerol addition on concentration of combustion products as well as temperature in heat exchanger have also been examined. The experimental results show that co-combustion of waste glycerol with wood sawdust does not worsen heating efficiency in a standard boiler. Moreover, 4.5 and 7% presence of glycerol in wood pellets correlated to a nearly twofold decrease of NOx concentration in flue gas. Therefore, the use of the waste glycerol as a binder for the production of pellets can be a simple and cost-effective solution of its utilization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Currently, in European Union, 8% addition of biodiesel to diesel fuel is universally applied and according to Directive 2009/28/EC, at the latest in 2020, 10% addition will become mandatory. Therefore, in recent years there has been a significant increase in the production of liquid biofuels derived from vegetable oils [1]. The plant source of biodiesel usually depends on the crops amenable to the regional climate. In South and North America, soybean oil is the most commonly biodiesel feedstock, whereas rapeseed oil is the most common source in European Union where its share of the total area of oilseed crops is somewhat more than 80% [2].
Biodiesel produced from rapeseed, called rape methyl ester (RME) is made using the transesterification process involving the methanolysis of rape seed oil [3, 4]. Aside from the main product, waste (crude) glycerol is also formed and it is generated always in amounts greater than 10% in relation to the RME produced [5,6,7]. Biodiesel enjoys increasing popularity. However, the dynamic growth of its production results of large amounts of the waste glycerol which constitutes a major obstacle to the profitability of the process.
So far, the most common method of waste glycerol utilizing has come down to its purification to pharmaceutical glycerin which finds numerous applications, e.g. in the pharmaceutical-, food-, or cosmetics industries [8,9,10]. In number of recently published studies one can meet several new alternatives for the use of the waste glycerol, e.g. conversion of glycerol into commodity chemicals (hydrogen, ethanol and methanol production, acrolein, organic acids and many others) [11,12,13,14,15,16]. Some authors also propose the use of glycerol as a feed additive for animals [17, 18], as an addition to automotive fuels [19] and for denitrification in waste water treatment plants [20, 21]. Unfortunately, the mentioned methods require preliminary purification of crude glycerol which adds costs. The simplest and the cheapest method of waste glycerol utilization seems to be its use for heat-generation [7, 22,23,24]. Glycerol reveals a relatively high calorific value (16.1–22.6 MJ/kg depending on the raw material used to biodiesel production [10, 13, 25]). Table 1 shows the heat of combustion of crude glycerol and feedstock from transesterification process obtained from different raw materials according to Thompson and He [25].
However, extremely high viscosity and relatively high flash-point disallow the use of waste glycerol as individual fuel, unless it is co-combusted with other fuels [10, 26,27,28,29]. Co-combustion of the waste glycerol with liquid- or gas fuels could be a solution, because, besides the benefits of waste management, it also contributes to the reduction of harmful combustion products. Unfortunately, the co-combustion of the crude glycerol with liquid fuels is always accompanied with the necessity of the costly modifications to the heating equipment [26]. Contrary, the co-combustion of crude glycerol with solid fuels, such as biomass, could be a cost-effective alternative without necessity of substantial modifications of the heating devices. Unfortunately, there is shortage of systematic literature reports relating the efficiency of co-firing of biomass with glycerol. In some papers a possibility of glycerol application as an addition to pellets is discussed.
For example, Brady et al. [30] consider a possibility of manufacturing of biomass pellets containing approximately 40% of glycerin. The authors have placed the pellets inside a rolled piece of newspaper wrapping. Although the cited authors established calorific value of obtained pellets to be on the level of 15 MJ/kg, they did not analyze mechanism of pellets combustion process. Bartocci et al. [31] described the co-combustion of waste glycerol with biomass in pyrolysis process. The authors found that the optimal percentage of glycerol is about 20% or lower. Similar conclusions were presented by Skoulou and Zabaniotou [32] for co-gasification of crude glycerol with lignocellulosic biomass.
In recent times, environmentally friendly methods of heating with the use of domestic biomass boilers utilizing wood pellets become increasingly popular. Biomass boilers are becoming more and more modern, allow for acceptable combustion efficiency and are automated. In addition, fuels such as wood and biomass are broadly available and relatively cheap [33,34,35]. The benefits of using biomass to displace coal are high greenhouse gases displacement and combustion is a high efficiency use of biomass. Because for the production of pellets moisture content of 8 to 18% is normally retained, to enhance binding it is reasonable to consider glycerol instead of some of the moisture content in the wood which can contribute to a higher calorific value of pellets. The role of briquetting process in terms of presence of binding components on burning efficiency has been discussed in Refs [36, 37].
Further development of biomass burning seems also to be an important challenge in face of progressive limitation of fossil fuels (and global security of energy supplies) as well as environmental and climatic regulations on CO2 emissions [38,39,40].
In this study, a possibility of the utilization of waste glycerol as an addition to wood pellets intended for heating purposes has been investigated. The pellets containing 2, 4.5 or 7 mass percent of glycerol additions have been compared with the applicable quality standards for wood pellets. Effect of crude glycerol addition in view of concentration of harmful combustion products has also been examined.
Materials and Methods
Materials: Specimens Preparation
The waste glycerol used in the study has been delivered by Trzebinia Refinery (RT S.A.), Poland. Crude glycerin obtained in the transesterification process has been subjected to preliminary purification consisting in its neutralization with sulfuric acid and separation of free fatty acids, potassium sulphate, unreacted methanol and water. The minimum content of glycerin in the pre-purified waste glycerol (called also a technical glycerin) was 80 mass%, ash content—below 5 mass%, matter organic non glycerol—below 6 mass% and the balance being water. Calorific value of the waste glycerol tested was 22.6 MJ/kg [10]. As the solid component, a dry spruce and pine wood sawdust (nearly 93 mass % of carbohydrates, mainly cellulose) with fragmentation of 2–4 mm and a bulk density of 180 kg/m3 has been used. Cylindrical, 5–15 mm long pellets, 6 ± 1 mm in diameter, have been fabricated with the use of MGL-200 pellets device, applying the wood sawdust without- and with 2, 4.5 or 7 mass% of waste glycerol additions. Larger amounts of waste glycerol in pellets caused significant disintegration and crushing of pellets during their production, therefore, a 7% glycerol addition was the maximum that was used. Pellets containing greater amounts of glycerol (7%) have also shown increased susceptibility to crumbling during their pouring. However, irrespectively of glycerol content, the pellet diameters were not less than 5 mm. The pellets bulk density was between 500 and 600 kg/m3 which falls into the limits of SS187120. In Fig. 1, a view of obtained pellets is presented—one can see a small effect of glycerol presence on average length of the pellets: the composite pellets (containing glycerol) are generally a little shorter than glycerol free pellets. The abbreviations “CP” (cellulose pellets) and “CGP” (cellulose/glycerin pellets) are applied to name the as received- and composite pellets tested. The glycerol mass percentage in the pellets is identified at the end of pellets symbol (e.g. CGP-2 symbol corresponds to 2 mass% of glycerol). The pressure used to compact the components was approx. 4 MPa.
Calorific values (combustion enthalpies) of obtained pellets have been determined using KL-12Mn calorimeter, PRECYZJA-BIT, Poland. The humidity of the tested pellets has been determined on the basis of 1.00 g pellets specimen mass loss after its drying in a temperature of 378 ± 5 K to constant mass. The ash content has been evaluated by specimen given mass burning and its air roasting at 873 ± 5 K to constant mass. For each kind of fuel the contents of carbon [C], hydrogen [H], nitrogen [N], chlorine [Cl], phosphorus [P] and sulfur [S] have been determined. The elemental composition of the tested pellets has been tested by infrared absorption method using S.C-632 and CHN-628 (LECO) analyzers.
The resulting pellets parameters (diameter, bulk density, moisture- and ash content, and calorific value) are well matched with the German (DIN51731) and Swedish (SS187120) standards. To evaluate the permitted emissions for the analyzed fuels the EN 303-5 standard [41] has been adopted.
Test Stand Description and Experimental Procedure
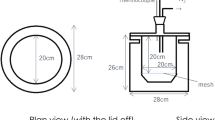
A 10 kW domestic biomass boiler, Mini Bio type, Kostrzewa (Poland) has been employed for pellets burning. The boiler has been equipped with a burner adapted for combustion of wood pellets having a diameter from 6 to 8 mm. The combustion tests were carried out using four types of pellets: CP, CGP-2, CGP-4.5 and CGP-7. In each experiment the same volume of fuel pellets (0.0075 m3 of known mass) has been combusted.
The boiler water reservoir was installed around the combustion chamber and was equipped with a thermostat for keeping water in given temperature range. The temperature of the thermostat during pellets combustion was set at 333 K. The surrounding temperature changes have been registered in 10 cm distance from the outer boiler wall. The starting surrounding temperature was checked directly before burning and settled at 296 ± 2 K for all tests. Changes of both ambient temperature and the water temperature in the boiler tank were measured using NiCr-NiAl thermocouples and registered versus waste glycerol content in the applied cellulosic fuel. Additionally, changes of temperature in the heat exchanger were monitored during combustion process using a thermovision camera at the point of water inlet to the heat exchanger.
During combustion of each of the chosen fuel compositions, continuous chemical analysis of flue gas was being carried out using Vario-Plus analyzer. The analyzer probe (number 7 in Fig. 2) was placed in a funnel, approx. 20 cm from the exhaust point. The applied gas analysis device utilized both electrochemical (O2 analysis) and infrared sensors (CO, SO2, NOx and CO2 analysis). The simplified scheme of the test stand is presented in Fig. 2.
Results and Discussion
Efficiency of Glycerol Co-combustion
The physicochemical characterization of the tested pellets is presented in Table 2.
The results of pellets elemental analysis are summarized in Table 3.
Results in Table 2 show that addition of glycerol contributes to only insignificant reduction of pellets moisture. This is rather an unexpected result, since applied glycerol contained about 10% of water and it presumably results from hygroscopic properties of glycerin. After the combustion of each kind of analyzed pellets, the quantities of ash remaining were practically the same (and equal to 0.70 ± 0.10%). The calorific value of the waste glycerol (22.6 MJ/kg) is a little greater than that of wood sawdust utilized for pellets manufacturing (18.1 MJ/kg), therefore, the increasing additions of glycerol to the pellets give rise their slight calorific value increase.
The share of the waste glycerol in pellets does not significantly affect the quantitative changes of carbon, hydrogen and nitrogen concentrations in the final product (Table 3). However, with the increase of glycerol content in the pellets the small downward trend for the [C] and [N] and, at the same time, upward of [H] and [O] can be observed. The changes for [C], [H] and [O] can result from greater content of hydrogen in glycerol as compared to cellulose. The chlorine content in the pellets, although very low, markedly decreases with increasing share of glycerol in the cellulosic fuel. Although the changes of [Cl] are nearly within the limits of analysis accuracy, one cannot exclude a chemical reaction of glycerol with Cl-containing compounds to form products undetectable by the applied analytical method (the method is sensitive to Cl− ions only). According to our earlier examinations [42], the decrease of Cl− content in the pellets, being a result of glycerol addition, is prone to lower corrosion aggressiveness of the flue gas condensates and has been observed towards steels which are most commonly applied of funnel pipes and chimney corners. For all kinds of analyzed fuels the sulfur and phosphorus contents were also very low: they did not exceed 0.02–0.03% which was also on the level of analysis accuracy.
The effect of waste glycerol content in the pellets on the average pellets consumption (defined as the amount of pellets (kg) combusted during 1 h) is illustrated in Fig. 3. It has been calculated on the basis of total combustion of 0.0075 m3 of given kind of fuel pellets, when setting the thermostat of the boiler on 333 K level.
As shown in Fig. 3, small addition of the waste glycerol into pellets causes increased rate of fuel consumption. As it has already been noted in Table 2, glycerol additions reduce pellets bulk density which may cause active surface development of the fuel pellets. Additionally, glycerol additions increase pellets lubricity. Therefore, average rate of the pellets consumption increases a little with small glycerol additions (2 and 4.5 mass%). The limitation of pellets consumption by greater additions of glycerol is observed for CGP-7 and can be ascribed to presence of water in the applied glycerol.
In Fig. 4 the ambient air temperature (Ta) and the boiler’s water temperature (Tb) changes recorded at consecutive firing/extinguishing cycles, are presented for the tested fuels. It should be added that temperature changes in the boiler were confirmed by thermovision camera evaluations in the heat exchanger.
Changes of water temperature in the boiler (Tb) during its operation with the use of the standard wood pellets (CP) and pellets containing 2% (CGP-2), 4.5% (CGP-4.5) and 7% (CGP-7) share of the waste glycerol. The experimental points denote resulting ambient temperature changes (Ta). (Color figure online)
It results from Fig. 4 that the length of firing cycles depends on type of pellets, whereas, according to expectations, the cooling rates are close to each other in case of all of the tested pellets. Generally, greater slopes of the “growing” segments (and, thus, greater heating rates) are observed for glycerol free- and for glycerol low (CGP-2) pellets. However, one should have been taken in mind that greater amounts of glycerol in the pellets (CGP-4.5 and CGP-7) result in shortening of time necessary for burning of the whole applied pellets volume (0.0075 m3). This seemingly contradictory effect results from markedly lower densities of glycerol-rich pellets (compare Table 2), and, consequently, 15–20% lower pellets mass in this volume. Despite the cycle time periods being the longest for greater glycerol contents, the efficiency of surrounding heating is comparable for all of the applied pellet types. It is seen in Fig. 4 that surrounding temperatures are slightly greater (by about 2–3 K) in case of CP. This difference should be ascribed to the actual experimental conditions. In particular, the starting surrounding temperatures were 297.7 K (in case of CP burning) and 293.9–294.7 K (in case of tests with CGP).
Effect of Glycerol Co-combustion on Gaseous Products Content in the Flue Gas
Concentrations of NOx, CO, CO2 and O2 in the flue gas obtained for CP and CGP are presented in Fig. 5.
As shown in Fig. 5, the NOx concentration of CP and CGP-2 is larger than the NOx concentration of CGP-4.5 and CGP-7 approximately in two times. This advantageous phenomenon can be ascribed to the decrease of fuel spent rate for glycerol-rich compositions (and, thus, decrease of burning temperature). As a consequence, the yield of the endothermic reaction of NOx formation decreases. The lowering of burning rate by greater glycerol amounts in the pellets (e.g. CGP-7) has also its implication in certain limitation of CO2 emission and comparatively high concentration of O2 in flue gas. Finally, the greater amounts of glycerol favor generation of CO in flue gas which is a harmful occurrence. Fortunately, the registered CO concentration is fairly low (and close to the analysis accuracy): 1900 ppm for CP and 2500 ppm for GCP-7. It must be emphasized that emissions of both CO and NOx recorded for the wood pellets modified with glycerol are nearly an order of magnitude lower than limiting emission values predicted by normative regulations.
Conclusions
The study indicates that the physicochemical parameters of resulting glycerin-wood pellets (with analyzed waste glycerol added volumes) meet the European standards. Co-combustion of the waste glycerol with wood sawdust does not worsen heating efficiency in a standard boiler. The small additions (up to 4.5%) of waste glycerol into wood pellets increase a little the pellets spend rate, at the same time, the 7% glycerol addition causes lowering of pellets consumption.
The results also show that co-combustion of pellets with the waste causes the decrease of concentration of NOx in flue gas. The glycerol additions have practically no effect on other harmful flue gas emissions.
For pellets containing 7% of glycerol a greater susceptibility to crushing and breaking takes place. Therefore, due to advantageous relation between combustion time and pellets density, as the most recommended content of waste glycerol in wood pellets it should be assumed to be 4.5% addition.
Having in mind energy balance, pellets consumption spent as well as limitation of NOx liberation, the use of waste glycerol as a binder for the production of wood pellets seems to be a very promising solution. Although additional environmental and economic analysis is required, it can be also considered as a cost-effective solution.
References
Vasudevan, P.T., Fu, B.: Environmentally sustainable biofuels: advances in biodiesel research. Waste Biomass Valor. 1(1), 47–63 (2010)
Singh, S.P., Singh, D.: Biodiesel production through the use of different sources and characterization of oils and their esters as the substitute of diesel: a review. Renew Sust Energy Rev. 14, 200–216 (2010)
Demirbas, A.: Biodiesel: a realistic fuel alternative for diesel engines. Springer, London (2008)
Tarakowski, R., Malanowski, A., Rostocki, A.J., Kowalczyk, M., Modzelewski, P., Ptasznik, S., Siegoczyński, R.M.: Could RME biodiesel be potentially harmful to modern engine? solidification process in RME. Fuel 146, 28–32 (2014)
Liang, Y.N., Cui, Y., Trushenski, J., Blackburn, J.W.: Converting crude glycerol derived from yellow grease to lipids through yeast fermentation. Bioresour. Technol. 101, 7581–7586 (2010)
Bohon, M.D., Metzger, B.A., Linak, W.P., King, C.J., Roberts, W.L.: Glycerol combustion and emissions. Proc. Combust. Inst. 33, 2717–2724 (2011)
Manara, P., Zabaniotou, A.: Co-valorization of crude glycerol waste streams with conventional and/or renewable fuels for power generation and industrial symbiosis perspectives. Waste Biomass Valor. 7(1), 135–150 (2016)
Singhabhandhu, A., Tezuka, T.: A perspective on incorporation of glycerin purification process in biodiesel plants using waste cooking oil as feedstock. Energy 35, 2493–2504 (2010)
Yang, F., Hanna, M.A., Sun, R.: Value-added uses for crude glycerol: a byproduct of biodiesel production. Biotechnol. Biofuels 5, 13 (2012)
Bala, A., Radomiak, H.: Possibilities for the utilization of waste glycerine as a fuel. COW 9, 365–369 (2012)
Leoneti, A., Aragão-Leoneti, V., Borges de Oliveira, S.: Glycerol as a by-product of biodiesel production in Brazil: Alternatives for the use of unrefined glycerol. Renew. Energy 45, 138–145 (2012)
Posada, J., Cardona, C.: Design and analysis of fuel ethanol production from raw glycerol. Energy 35, 5286–5293 (2010)
Quispe, C.A.G., Coronado, C.J.R., Carvalho, J.A. Jr.: Glycerol: production, consumption, prices, characterization and new trends in combustion. Renew. Sust. Energy Rev. 27, 475–493 (2013)
Skaf, D.W., Natrin, N.G., Brodwater, K.C., Bongo, C.R.: Comparison of photocatalytic hydrogen production from glycerol and crude glycerol obtained from biodiesel processing. Catal. Lett. 142, 1175–1179 (2012)
Ntaikou, I., Antonopoulou, G., Lyberatos, G.: Biohydrogen production from biomass and wastes via dark fermentation: a review. Waste Biomass Valor. 1(1), 21–39 (2010)
Liebminger, S., Hofbauer, R., Siebenhofer, M., Nyanhongo, G.S., Guebitz, G.M.: Microbial conversion of crude glycerol to dihydroxyacetone. Waste Biomass Valor. 5(5), 781–787 (2014)
Donkin, S.S., Koser, S.L., White, H.M., Doane, P.H., Cecava, M.J.: Feeding value of glycerol as a replacement for corn grain in rations fed to lactating dairy cows. J. Dairy Sci. 92, 5111–5119 (2009)
Nitayavardhana, S., Khanal, S.K.: Biodiesel-derived crude glycerol bioconversion to animal feed: a sustainable option for a biodiesel refinery. Bioresour. Technol. 102, 5808–5814 (2011)
Karinen, R.S., Krause, A.O.I.: New biocomponents from glycerol. Appl. Catal. 306, 128–133 (2006)
Bodík, I., Blstakova, A., Sedlácek, S., Hutnan, M.: Biodiesel waste as source of organic carbon for municipal WWTP denitrification. Bioresour. Technol. 100(8), 2452–2456 (2009)
Fountoulakis, M.S., Petousi, I., Manios, T.: Co-digestion of sewage sludge with glycerol to boost biogas production. Waste Manag. 30, 1849–1853 (2010)
Gupta, M., Kumar, N.: Scope and opportunities of using glycerol as an energy source. Renew Sust Energy Rev. 16, 4551–4556 (2012)
Steinmetz, S.A., Herrington, J.S., Winterrowd, C.K., Roberts, W., Wendt, J., Linak, W.: Crude glycerol combustion: particulate, acrolein, and other volatile organic emissions. Proc. Combust. Inst. 34, 2749–2757 (2013)
Patzer, R.: Stack emissions evaluation: combustion of crude glycerin and yellow grease in an industrial fire tube boiler. Agricultural Utilization Research Institute Marshall, Marshall (2007)
Thompson, J.C., He, B.B.: Characterization of crude glycerol from biodiesel production from multiple feed stocks. Appl. Eng. Agric. 22, 261–265 (2006)
Queirós, P., Costa, M., Carvalho, R.H.: Co-combustion of crude glycerin with natural gas and hydrogen. Proc. Combust. Inst. 34, 2759–2767 (2013)
Jiang, L., Agrawa, A.K.: Combustion of straight glycerol with/without methane using a fuel-flexible, low-emissions burner. Fuel. 136, 177–184 (2014)
Bala-Litwiniak, A., Radomiak, H.: Environmental benefits of co-combustion of light fuel oil with waste glycerol. Energy Sources A 38(17), 2510–2516 (2016)
Sakkampang, C., Wongwuttanasatian, T.: Study of ratio of energy consumption and gained energy during briquetting process for glycerin-biomass briquette fuel. Fuel 115, 186–189 (2014)
Brady, S., Tam, K., Leung, G., Salam, C.: Zero waste biodiesel: using glycerine and biomass to create renewable energy. Undergrad. Res. J. 2, 5–11 (2008)
Bartocci, P., Bidini, G., Asdrubali, F., Beatrice, C., Frusteri, F., Fantozzi, F.: Batch pyrolysis of pellet made of biomass and crude glycerol: mass and energy balances. Biores. Technol. 249, 473–478 (2018)
Skoulou, V.K., Zabaniotou, A.A.: Co-gasification of crude glycerol with lignocellulosic biomass for enhanced syngas production. J. Anal. Appl. Pyrol. 99, 110–116 (2013)
Walker, N.: Biomass: fueling change. Crabtree Publishing, Crabtree (2007)
Serrano, C., Portero, H., Monedero, E.: Pine chips combustion in a 50 kW domestic biomass boiler. Fuel 111, 564–573 (2013)
Sefidari, H., Razmjoo, N., Strand, M.: An experimental study of combustion and emissions of two types of woody biomass in a 12-MW reciprocating-grate boiler. Fuel 135, 120–129 (2014)
Kosturkiewicz, B., Janewicz, A., Magdziarz, A.: Results of briquetting and combustion process on binder-free coking coal. Pol. J. Environ. Studies. 23, 1385–1389 (2014)
Magdziarz, A., Kuźnia, M., Bembenek, M., Gara, P., Hryniewicz, M.: Briquetting of EAF dust for its utilisation in metallurgical processes. Chem. Process Eng. 36(2), 263–271 (2015)
Magdziarz, A., Wilk, M., Zajemska, M.: Modelling of pollutants concentrations from the biomass combustion process. Chem. Proc. Eng. 32, 423–433 (2011)
Zajemska, M., Musiał, D., Radomiak, H., Poskart, A., Wyleciał, T., Urbaniak, D.: Formation of pollutants in the process of co-combustion of different biomass grades. Pol. J. Environ. Stud. 23, 1445–1448 (2014)
Radomiak, H., Zajemska, M., Musial, D., Poskart, A., Wylecial, T.: Forecasting of selected environmental hazards from combustion processes. Przem Chem. 96, 1466–1471 (2017)
EN 303-5 European Standard Heating boilers: part 5—heating boilers for solid fuels, manually and automatically stoked, nominal heat output of up to 500 kW—terminology, requirements, testing and marking (2002)
Bala-Litwiniak, A., Kamieniak, K.: Corrosion resistance of the boiler- and chimney steels in the exhaust fume condensates coming from burning of cellulose-waste glycerin based compositions. Ochr przed Koroz 57, 179–183 (2014)
Acknowledgements
The work was supported by Ministry of Science and Higher Education of Poland, within statutory research No. BS/PB-205-301/2006/P.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Bala-Litwiniak, A., Radomiak, H. Possibility of the Utilization of Waste Glycerol as an Addition to Wood Pellets. Waste Biomass Valor 10, 2193–2199 (2019). https://doi.org/10.1007/s12649-018-0260-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-018-0260-7