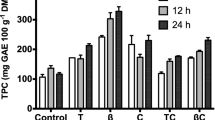
Abstract
This study demonstrated increases in the recovery of phenolic compounds from industrial waste, longan peel, after treatment with plant matrix degrading enzymes. Pretreatment of longan peel with cellulase, α-amylase, protease, or β-glucosidase enhanced total phenolic content in the extracts, leading to increases of DPPH scavenging activity, oxygen radical absorbance capacity, antioxidant in β-carotene/linoleate system, and ferrous ion chelating activity. In detail, cellulase increased the release of free phenolics e.g. o-coumaric acid, corilagin, and quercetin as well as the release of esterified phenolics e.g. ellagic acid, and gallic acid, while β-glucosidase increased the release of free phenolics e.g. ellagic acid. Among all enzymatic pretreatments, protease provided the highest proportion of phenolics’ recovery in the extract, while only cellulase and β-glucosidase significantly improved extraction yields by 31 and 17%, respectively. These results indicated enhancement of valorization of longan peel as a resource material for functional ingredients recovery.
Graphic Abstract





Similar content being viewed by others
Abbreviations
- DM:
-
Dry matter
- GAE:
-
Gallic acid equivalent
- TE:
-
Trolox equivalent
- EDTAE:
-
EDTA equivalent
- ORAC:
-
Oxygen radical absorbance capacity
- AAC:
-
Antioxidant activity coefficient
- CE:
-
Cellulase-assisted extraction
- AE:
-
α-Amylase-assisted extraction
- PE:
-
Protease-assisted extraction
- GE:
-
β-Glucosidase-assisted extraction
- CGE:
-
Cellulase and β-glucosidase-assisted extraction
- AGE:
-
α-Amylase and β-glucosidase-assisted extraction
- CAPE:
-
Cellulase, α-amylase and protease-assisted extraction
- CAGE:
-
Cellulase, α-amylase and β-glucosidase-assisted extraction
- CAPGE:
-
Cellulase, α-amylase protease and β-glucosidase-assisted extraction
References
Nguyen, V.T., Khong, T.T.: Economics and market for recovered bioactive compounds from agricultural wastes. In: Nguyen, V.T. (ed.) Recovering bioactive compounds from agricultural wastes, 1st edn, pp. 221–249. Wiley (2017)
Qiu, D.: Longan production and research in China. In: IV International Symposium on Lychee, Longan and Other Sapindaceae Fruits 2012, pp. 39–46
Subhadrabandhu, S., Yapwattanaphun, C.: Lychee and longan production in Thailand. In: International Symposium on Litchi and Longan 2001, pp. 49–57. Acta Horticulturae
Choo, W.K.: Longan production in Asia. Food and agriculture organization of the United Nations, regional office for Asia and the Pacific, RAP PUBLICATION: 2000/2020 (2000)
Sruamsiri, S., Silman, P.: Chemical composition and in vitro digestibility of by-products from longan production. J. Agric. Res. Ext. 32(2), 50–58 (2015)
He, N., Wang, Z., Yang, C., Lu, Y., Sun, D., Wang, Y., Shao, W., Li, Q.: Isolation and identification of polyphenolic compounds in longan pericarp. Sep. Purif. Technol. 70(2), 219–224 (2009)
Jaitrong, S., Rattanapanone, N., Manthey, J.A.: Analysis of the phenolic compounds in longan (Dimocarpus longan Lour.) peel. In: Proceedings of the Florida State Horticultural Society 2006, pp. 371–375
Rangkadilok, N., Worasuttayangkurn, L., Bennett, R.N., Satayavivad, J.: Identification and quantification of polyphenolic compounds in longan (Euphoria longana Lam.) fruit. J. Agric. Food. Chem. 53(5), 1387–1392 (2005)
Rerk-am, U.: Anti-oxidant activities and polyphenolic compounds of longan (Dimocarpus longan Lour) peel and seed extracts. Thai J. Pharm. Sci. (TJPS) 40, 120–122 (2016)
Huang, G.-J., Wang, B.-S., Lin, W.-C., Huang, S.-S., Lee, C.-Y., Yen, M.-T., Huang, M.-H.: Antioxidant and anti-inflammatory properties of longan (Dimocarpus longan Lour.) pericarp. Evid. Based Complementary Altern. Med. 1, 10 (2012). https://doi.org/10.1155/2012/709483
Li, L., Xu, J., Mu, Y., Han, L., Liu, R., Cai, Y., Huang, X.: Chemical characterization and anti-hyperglycaemic effects of polyphenol enriched longan (Dimocarpus longan Lour.) pericarp extracts. J. Funct. Foods 13, 314–322 (2015)
Ripa, F.A., Dash, P.R., Nesa, M.L., Sheikh, Z.: Phytochemical and hypoglycemic screening of seeds and peel of Nephelium longan fruits. Int. J. Biochem. 197, 483–489 (2015)
Ripa, F.A., Haque, M., Bulbul, I.J., Begum, Y., Habib, A.: Screening of central nervous system (CNS) depressant and antinociceptive activities of methanolic extracts of the peel and seed of Nephelium longan fruits. Afr. J. Pharm. Pharmacol. 6(11), 848–854 (2012)
Alam Ripa, F., Habib, A.: Anti-inflammatory and anti-diarrheal effects of methanolic extracts of seeds and peel of Nephelium longan fruits in rats. Iran. J. Pharmacol. Ther. 12(2), 58 (2014)
Prasad, K.N., Hao, J., Shi, J., Liu, T., Li, J., Wei, X., Qiu, S., Xue, S., Jiang, Y.: Antioxidant and anticancer activities of high pressure-assisted extract of longan (Dimocarpus longan Lour.) fruit pericarp. Innov. Food Sci. Emerg. Technol. 10(4), 413–419 (2009)
Rangkadilok, N., Sitthimonchai, S., Worasuttayangkurn, L., Mahidol, C., Ruchirawat, M., Satayavivad, J.: Evaluation of free radical scavenging and antityrosinase activities of standardized longan fruit extract. Food Chem. Toxicol. 45(2), 328–336 (2007)
Ahmad, N., Zuo, Y., Lu, X., Anwar, F., Hameed, S.: Characterization of free and conjugated phenolic compounds in fruits of selected wild plants. Food Chem. 190, 80–89 (2016)
Chandrasekara, A., Shahidi, F.: Determination of antioxidant activity in free and hydrolyzed fractions of millet grains and characterization of their phenolic profiles by HPLC-DAD-ESI-MSn. J. Funct. Foods 3(3), 144–158 (2011)
Barberousse, H., Roiseux, O., Robert, C., Paquot, M., Deroanne, C., Blecker, C.: Analytical methodologies for quantification of ferulic acid and its oligomers. J. Sci. Food Agric. 88(9), 1494–1511 (2008)
White, B.L., Howard, L.R., Prior, R.L.: Release of bound procyanidins from cranberry pomace by alkaline hydrolysis. J. Agric. Food. Chem. 58(13), 7572–7579 (2010)
Apolinar-Valiente, R., Romero-Cascales, I., Gómez-Plaza, E., Ros-García, J.M.: Degradation of Monastrell grape skins: effect of individual enzymatic activities and their synergic combination. Eur. Food Res. Technol. 243(11), 1933–1942 (2017)
Xu, C., Yagiz, Y., Borejsza-Wysocki, W., Lu, J., Gu, L., Ramírez-Rodrigues, M.M., Marshall, M.R.: Enzyme release of phenolics from muscadine grape (Vitis rotundifolia Michx.) skins and seeds. Food Chem. 157, 20–29 (2014)
Xu, L., He, W., Lu, M., Yuan, B., Zeng, M., Tao, G., Qin, F., Chen, J., Guan, Y., He, Z.: Enzyme-assisted ultrasonic-microwave synergistic extraction and UPLC-QTOF-MS analysis of flavonoids from Chinese water chestnut peels. Ind. Crops Prod. 117, 179–186 (2018)
Zhang, G., Hu, M., He, L., Fu, P., Wang, L., Zhou, J.: Optimization of microwave-assisted enzymatic extraction of polyphenols from waste peanut shells and evaluation of its antioxidant and antibacterial activities in vitro. Food Bioprod. Process. 91(2), 158–168 (2013)
Jiang, G., Jiang, Y., Yang, B., Yu, C., Tsao, R., Zhang, H., Chen, F.: Structural characteristics and antioxidant activities of oligosaccharides from longan fruit pericarp. J. Agric. Food Chem. 57(19), 9293–9298 (2009)
Wanlapa, S., Wachirasiri, K., Sithisam-ang, D., Suwannatup, T.: Potential of selected tropical fruit peels as dietary fiber in functional foods. Int. J. Food Prop. 18(6), 1306–1316 (2015)
del Pilar Sánchez-Camargo, A., Montero, L., Stiger-Pouvreau, V., Tanniou, A., Cifuentes, A., Herrero, M., Ibáñez, E.: Considerations on the use of enzyme-assisted extraction in combination with pressurized liquids to recover bioactive compounds from algae. Food Chem. 192, 67–74 (2016)
Hong, Y.-H., Jung, E.Y., Park, Y., Shin, K.-S., Kim, T.Y., Yu, K.-W., Chang, U.J., Suh, H.J.: Enzymatic improvement in the polyphenol extractability and antioxidant activity of green tea extracts. Biosci. Biotechnol. Biochem. 77(1), 22–29 (2013)
Zhou, Z., Shao, H., Han, X., Wang, K., Gong, C., Yang, X.: The extraction efficiency enhancement of polyphenols from Ulmus pumila L. barks by trienzyme-assisted extraction. Ind. Crops Prod. 97, 401–408 (2017)
Lin, S., Wang, Z., Hu, J., Ge, S., Zheng, B., Zeng, S.: Polyphenolics from fresh lotus seeds: enzyme-assisted ethanol extraction optimization and its antioxidant activity. Curr. Top. Nutraceutical. Res. 16(1), 85–96 (2018)
Chen, P.X., Bozzo, G.G., Freixas-Coutin, J.A., Marcone, M.F., Pauls, P.K., Tang, Y., Zhang, B., Liu, R., Tsao, R.: Free and conjugated phenolic compounds and their antioxidant activities in regular and non-darkening cranberry bean (Phaseolus vulgaris L.) seed coats. J. Funct. Foods 18, 1047–1056 (2015)
Singleton, V.L., Rossi, J.A.: Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 16(3), 144–158 (1965)
Brand-Williams, W., Cuvelier, M.-E., Berset, C.: Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 28(1), 25–30 (1995)
Huang, D., Ou, B., Hampsch-Woodill, M., Flanagan, J.A., Prior, R.L.: High-throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format. J. Agric. Food Chem. 50(16), 4437–4444 (2002)
Jayaprakasha, G., Singh, R., Sakariah, K.: Antioxidant activity of grape seed (Vitis vinifera) extracts on peroxidation models in vitro. Food Chem. 73(3), 285–290 (2001)
Carter, P.: Spectrophotometric determination of serum iron at the submicrogram level with a new reagent (ferrozine). Anal. Biochem. 40(2), 450–458 (1971)
Chandrasekara, A., Shahidi, F.: Content of insoluble bound phenolics in millets and their contribution to antioxidant capacity. J. Agric. Food Chem. 58(11), 6706–6714 (2010)
Patthawaro, S., Lomthaisong, K., Saejung, C.: Bioconversion of agro-industrial waste to value-added product lycopene by photosynthetic bacterium Rhodopseudomonas faecalis and its carotenoid composition. Waste Biomass Valoriz. (2019). https://doi.org/10.1007/s12649-018-00571-z
Llano, T., Alexandri, M., Koutinas, A., Gardeli, C., Papapostolou, H., Coz, A., Quijorna, N., Andres, A., Komaitis, M.: Liquid–liquid extraction of phenolic compounds from spent sulphite liquor. Waste Biomass Valorization 6(6), 1149–1159 (2015)
Wang, J., Zhao, L.-J., Zhang, Y.-Q.: UV irradiation and salicylic acid immersion enhance the level of Mulberroside a in isolated mulberry branches—a huge amount of agro-waste. Waste Biomass Valorization 8(8), 2643–2651 (2017)
Puls, J.: Chemistry and biochemistry of hemicelluloses: Relationship between hemicellulose structure and enzymes required for hydrolysis. In: Macromolecular Symposia 1997, vol. 1, pp. 183–196. Wiley Online Library
Singhania, R.R., Patel, A.K., Sukumaran, R.K., Larroche, C., Pandey, A.: Role and significance of beta-glucosidases in the hydrolysis of cellulose for bioethanol production. Biores. Technol. 127, 500–507 (2013)
Prasad, K., Yang, B., Zhao, M., Sun, J., Wei, X., Jiang, Y.: Effects of high pressure or ultrasonic treatment on extraction yield and antioxidant activity of pericarp tissues of longan fruit. J. Food Biochem. 34(4), 838–855 (2010)
Prasad, K.N., Yang, B., Shi, J., Yu, C., Zhao, M., Xue, S., Jiang, Y.: Enhanced antioxidant and antityrosinase activities of longan fruit pericarp by ultra-high-pressure-assisted extraction. J. Pharm. Biomed. Anal. 51(2), 471–477 (2010)
Pan, Y., Wang, K., Huang, S., Wang, H., Mu, X., He, C., Ji, X., Zhang, J., Huang, F.: Antioxidant activity of microwave-assisted extract of longan (Dimocarpus Longan Lour.) peel. Food Chem. 106(3), 1264–1270 (2008)
Wang, L., Wu, Y., Liu, Y., Wu, Z.: Complex enzyme-assisted extraction releases antioxidative phenolic compositions from guava leaves. Molecules 22(10), 1648–1662 (2017)
Mushtaq, M., Sultana, B., Anwar, F., Adnan, A., Rizvi, S.S.: Enzyme-assisted supercritical fluid extraction of phenolic antioxidants from pomegranate peel. J. Supercrit. Fluids 104, 122–131 (2015)
Mushtaq, M., Sultana, B., Akram, S., Anwar, F., Adnan, A., Rizvi, S.S.H.: Enzyme-assisted supercritical fluid extraction: an alternative and green technology for non-extractable polyphenols. Anal. Bioanal. Chem. 409(14), 3645–3655 (2017). https://doi.org/10.1007/s00216-017-0309-7
Shahidi, F., Varatharajan, V., Oh, W.Y., Peng, H.: Phenolic compounds in agri-food by-products, their bioavailability and health effects. J. Food Bioact. 5, 57–119 (2019)
de Camargo, A.C., Regitano-d’Arce, M.A.B., Biasoto, A.C.T., Shahidi, F.: Enzyme-assisted extraction of phenolics from winemaking by-products: Antioxidant potential and inhibition of alpha-glucosidase and lipase activities. Food Chem. 212, 395–402 (2016)
Norsker, M., Bloch, L., Adler-Nissen, J.: Enzymatic degradation of plant cell wall polysaccharides: the kinetic effect of competitive adsorption. Food/Nahrung 43(5), 307–310 (1999)
Kaya, F., Heitmann, J.A., Joyce, T.W.: Influence of lignin and its degradation products on enzymatic hydrolysis of xylan. J. Biotechnol. 80(3), 241–247 (2000)
Jodayree, S., Smith, J.C., Tsopmo, A.: Use of carbohydrase to enhance protein extraction efficiency and antioxidative properties of oat bran protein hydrolysates. Food Res. Int. 46(1), 69–75 (2012)
Bouchard, A., Hofland, G.W., Witkamp, G.-J.: Properties of sugar, polyol, and polysaccharide water–ethanol solutions. J. Chem. Eng. Data 52(5), 1838–1842 (2007)
Xu, M.-S., Chen, S., Wang, W.-Q., Liu, S.-Q.: Employing bifunctional enzymes for enhanced extraction of bioactives from plants: flavonoids as an example. J. Agric. Food Chem. 61(33), 7941–7948 (2013)
Liu, L., Wen, W., Zhang, R., Wei, Z., Deng, Y., Xiao, J., Zhang, M.: Complex enzyme hydrolysis releases antioxidative phenolics from rice bran. Food Chem. 214, 1–8 (2017)
Van Le, H.: Comparison of enzyme-assisted and ultrasound-assisted extraction of vitamin C and phenolic compounds from acerola (Malpighia emarginata DC.) fruit. Int. J. Food Sci. Technol. 47(6), 1206–1214 (2012)
Gómez-García, R., Martínez-Ávila, G.C., Aguilar, C.N.: Enzyme-assisted extraction of antioxidative phenolics from grape (Vitis vinifera L.) residues. 3 Biotech 2(4), 297–300 (2012)
Karadag, A., Ozcelik, B., Saner, S.: Review of methods to determine antioxidant capacities. Food Anal. Methods 2(1), 41–60 (2009). https://doi.org/10.1007/s12161-008-9067-7
Prior, R.L., Wu, X., Schaich, K.M.: Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 53(10), 4290–4302 (2005)
Laguerre, M., Bayrasy, C., Panya, A., Weiss, J., McClements, D.J., Lecomte, J., Decker, E.A., Villeneuve, P.: What makes good antioxidants in lipid-based systems? The next theories beyond the polar paradox. Crit. Rev. Food Sci. Nutr. 55(2), 183–201 (2015)
Shahidi, F., Zhong, Y.: Revisiting the polar paradox theory: a critical overview. J. Agric. Food Chem. 59(8), 3499–3504 (2011)
Wang, T., Jonsdottir, R., Ólafsdóttir, G.: Total phenolic compounds, radical scavenging and metal chelation of extracts from Icelandic seaweeds. Food Chem. 116(1), 240–248 (2009)
Sun, L., Zhang, H., Zhuang, Y.: Preparation of free, soluble conjugate, and insoluble-bound phenolic compounds from peels of rambutan (Nephelium lappaceum) and evaluation of antioxidant activities in vitro. J. Food Sci. 77(2), C198–C204 (2012)
Fujita, A., Alencar, S., Park, Y.: Conversion of isoflavone glucosides to aglycones by partially purified β-glucosidases from microbial and vegetable sources. Appl. Biochem. Biotechnol. 176(6), 1659–1672 (2015)
Newsome, A.G., Li, Y., Van Breemen, R.B.: Improved quantification of free and ester-bound gallic acid in foods and beverages by UHPLC-MS/MS. J. Agric. Food Chem. 64(6), 1326–1334 (2016)
Acknowledgement
This work was financially supported by “The National Natural Science Foundation of China (Grant No. 31871759)”and “Project of Distinguished Professor of Liaoning Province (2015-153)”. Some of the standard chemicals were kindly provided by Dr. Nuansri Rakariyatham from Nation University, Lampang, Thailand.
Funding
This work was funded by The National Natural Science Foundation of China (Grant No. 31871759) and Project of Distinguished Professor of Liaoning Province (2015-153).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
12649_2019_723_MOESM1_ESM.docx
Supplementary material 1 (DOCX 156 kb) Online resource 1 High performance liquid chromatography (HPLC) chromatograms at 270 nm of standard phenolics (A), and representative crude extracts of longan peel with and without enzymatic pretreatments (B): cellulase-assisted extraction (CE); α-amylase-assisted extraction (AE); protease-assisted extraction (PE); β-glucosidase-assisted extraction (GE). Peaks: 1, Gallic acid; 2, Chlorogenic acid; 3, Corilagin; 4, Ferulic acid; 5, Ellagic acid; 6, o-Coumaric acid; 7, Quercetin; 8, Kaempferol. Noted that ferulic acid and chlorogenic acid were quantified at 330 nm (chromatogram not shown)
Rights and permissions
About this article
Cite this article
Rakariyatham, K., Liu, X., Liu, Z. et al. Improvement of Phenolic Contents and Antioxidant Activities of Longan (Dimocarpus longan) Peel Extracts by Enzymatic Treatment. Waste Biomass Valor 11, 3987–4002 (2020). https://doi.org/10.1007/s12649-019-00723-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-019-00723-9