Abstract
Titanium dioxide nanoparticles (TiO2 NPs) are the most produced nanomaterial for food additives, pigments, photocatalysis, and personal care products. These nanomaterials are at the forefront of rapidly developing indispensable nanotechnology. In all these nanomaterials, titanium dioxide (TiO2) is the most common nanomaterial which is being synthesized for many years. These nanoparticles of TiO2 are widely used at the commercial level, especially in cosmetic industries. High usage in such a way has increased the toxicological consequences of the human population. Several studies have shown that TiO2 NPs accumulated after oral exposure or inhalation in the alimentary canal, lungs, heart, liver, spleen, cardiac muscle, and kidneys. Additionally, in mice and rats, they disturb glucose and lipid homeostasis. Moreover, TiO2 nanoparticles primarily cause adverse reactions by inducing oxidative stress that leads to cell damage, inflammation, genotoxicity, and adverse immune responses. The form and level of destruction are strongly based on the physical and chemical properties of TiO2 nanoparticles, which administer their reactivity and bioavailability. Studies give indications that TiO2 NPs cause both DNA strand breaks and chromosomal damages. The effects of genotoxicity do not depend only on particle surface changes, size, and exposure route, but also relies on the duration of exposure. Most of these effects may be because of a very high dose of TiO2 NPs. Despite increased production and use, epidemiological data for TiO2 NPs is still missing. This review discusses previous research regarding the impact of TiO2 NP toxicity on human health and highlights areas that require further understanding in concern of jeopardy to the human population. This review is important to point out areas where extensive research is needed; thus, their possible impact on individual health should be investigated in more details.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Nanoparticles are small materials ranging in size from 1 to 100 nm and can be classified into various categories depending on their shape and size [1]. These particles have unique physicochemical properties due to their large surface area and small size that makes this material excellent in many areas of human research activities, cosmetic products, and agriculture [2].
TiO2 is one of the most used nanoparticles nowadays. It is a white pigmented additive with high opacity and coating properties. It is generally used in dye, clothing, rubber, paper, ceramic, metallurgy, drug, cosmetic, pharmaceuticals, food industries, car materials, and other biomaterials for sterilization and industrial photolytic processes regarding the decomposing of organic matters [3]. These particles have several adverse effects at the cellular level, such as oxidative stress and DNA damage [4]. Meanwhile, oxidative stress is a significant determinant of nanoparticle (NPs) induced injuries. So, it is essential to illustrate the reactive oxygen species (ROS) response resulting from NPs [5]. Hence, increasing human exposure to nanoparticles has an increasing concern for their safety and health. In experimental animals, TiO2 cause severe pulmonary response [6]. The situation is more worse after turning into anatase form under UV irradiation [7]. It has been proved that TiO2 anatase form has more toxic properties than TiO2 rutile form, because smaller particles with larger surface enhance the side effects [8]. So, the overall safety of TiO2 NPs is still at the initial stage. It is generally known that TiO2 NPs have a higher biological activity than ordinary bulk materials due to their large surface area to volume ratio [8]. So, these unique NPs features raise concerns about human safety regarding health [9]. Therefore, additional efforts are still needed to understand the interaction between these NPs and the human body. In this regard, nanotoxicology and nanolithography attract the attention of toxicologists and regulatory scientists [6].
So, it is necessary to evaluate the adverse health effects and environmental biosafety of TiO2 NPs and highlights those areas where further understanding in concern of jeopardy to the human population is needed.
2 Physical Properties of Nanostructured TiO2
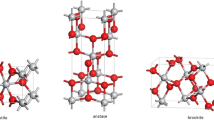
TiO2 is naturally found in various rocks and mineral sands. Naturally occurring TiO2 comes in a red or brownish red or black color. The color is usually due to the presence of impurities such as iron, vanadium, zirconium, and chromium, which can make up 10% of all titanium [10]. Titanium is the ninth common element in the Earth’s crust. Titanium dioxide belongs to the family of transition metal oxides. TiO2 is a white, odorless, nonflammable powder with a 79.9-g/mol molecular weight, a boiling point of 2972 °C, a melting point of 1843 °C, and a relative density of 4.26 g/cm3 at 25 °C. TiO2 is entirely insoluble in water, common organic solvent, and dilute acids. It is soluble in concentrated sulfuric acid or hydrofluoric acid at high temperatures [11]. TiO2 has excellent electrical properties due to its high dielectric constant. It does not react with oxygen, sulfur dioxide, hydrogen sulfide, ammonia, and carbon dioxide. TiO2 has chemical stability, biocompatibility, and a robust photocatalytic activity. It exists in three common polymorphs in nature, i.e., brookite, anatase, rutile, and a few common structures of TiO2 [12]. It is a wideband semiconductor with anatase, rutile, and brookite bandgap of 3.2, 3.02, and 2.96 eV, respectively [13]. The small size of TiO2 renders it more genetically toxic regardless of its crystalline levels. According to many studies, the small sizes of nanoparticles allow easy entry and accumulation inside the cell’s of cytoplasm and nucleus [14]. Several pieces of literature show that the anatase form of nanoparticles cause more toxic effects than rutile nanoparticles because the anatase form has more photocatalytic properties. TiO2 NPs produce large size agglomerates that cause DNA damage in different cell lines [15]. These small size nanoparticles express higher toxicity than large size particles. Moreover, the nanorods of TiO2 exhibit more toxicity than spherical particles having the same surface area and size, indicating the role toward cytotoxicity [16].
3 Mass Production and Filthiness of TiO2 NPs
In 2006, the USA manufactures 40,000 tons of TNPs [17]. Due to increased market demand, the annual production of TiO2 NPs is expected to reach 2.5 million tons by 2025 [18]. Therefore, a huge quantity of TiO2 NPs will be released into the environment (Fig. 1). The widespread distribution of TiO2 nanoparticles is released into the air, soil, and/or water throughout and can affect all components of the environment, including humans, animals, and plants on direct exposure to these pollutants [20]. Transfer in food chain usually happens from plants to animals, because plants are a primary food chain source that consumes nutrients and waste from the environment with toxins. Accumulation and translocation were studied by analytical tools to detect NPs in numerous plant tissues that trigger leaf necrosis, inhibit seedlings’ root elongation, and influence root growth [21]. However, ecological life examinations, potential bioaccumulation, and especially the transfer of NPs within the food chain and air remain restricted. Because TiO2 NPs are inevitable in the production, use, and disposal of waste through the air, soil, and water, the natural environment’s ecological environment has attracted considerable attention both domestically and internationally [22]. Employees and academic researchers of production industries may experience the highest threat to exposure of these NPs through inhalation and skin penetration [11]. Wastewater treatment plants contain 100–3000g TiO2 NPs with a ratio of 5–15g Ti/L [23]. Most countries standardize solid waste disposal, but non exclusively addresses nanoparticle removal. Thus, the nanoparticle pollution hazard is important and cannot be unnoticed.
Possibility of release, absorption, and impact of TiO2 NPs in an ecosystem (1) inhalation; (2) ingestion; and (3) deposition [19]
4 Potential Toxicity of TiO2 NPs and Its Accumulation
Recently, growing interest in nanotechnology applications has been observed in various fields like agriculture, medicine, pharmacy, and materials science. Due to its crystal structure, size, and coating, use of TiO2 NP is inevitable. Particle size, crystalline structure, and coating affect the surface charge, agglomeration, and sedimentation, thereby making the TiO2 NP very toxic to human cells. Previous researches show that TiO2 NPs disturb glucose and lipid homeostasis in mice and rats [24]. Available data on TiO2 NP toxicity to humans is limited, so the potential risk is still in doubt. For that reason, investigators are using numerous toxicological models, such as human cells, animals, and aquatic organisms to generate desirable facts to avoid toxicity (Fig. 2).
Schematic diagram of the toxicity ofTiO2 NPs [25]
Previous in vitro and in vivo tests have confirmed the toxic effects of TiO2 NPs on the human body like altered cell cycle, nuclear stenosis, and apoptosis [26,27,28,29]. Studies have also shown that TiO2 NPs causes DNA damage and causes rupturing of the small intestine epithelium, which is involved in the absorption of nutrients [30]. This damage is due to various ways, mainly by inhalation, injection, and skin contact, as well as digestion and absorption [11]. In print plant manufacturing units, workers were found to have symptoms such as shortness of breath because they were exposed to polyacrylate nanoparticles coupled with TiO2 NPs without any protective procedures [31]. Other clinical signs of TiO2 toxicity may include rashes on the face, hands, and forearms [32], and pleural effusion [33]. Some even suffered from pericardial effusions [34], hypoxemia [31], and cancer [35,36,37]. In vivo testing of such exposure revealed that inhalation or oral exposure of TiO2 NPs may accumulate in different places like the liver, heart, spleen, lungs, kidneys, alimentary tract, and cardiac muscle (Table 1) [47,48,49].
5 Bio Distribution and Systemic Toxicity in the Different Organ System
All nanomaterials can differ considerably in composition, charge, morphology, specific surface area, and state of matter, which influences different organs (Fig. 3) [51] and may be found in the lung, kidney, lymph node, liver, and spleen [52]. TiO2 NPs can be transported through the digestive tract to other organs or tissues, which can lead to liver damage and myocardial damage [53].
The dynamic nature of NPs in vivo. Radiation penetrates the systemic circulation. The black line represents the nanoparticle confirmation path, and the dotted line represents the virtual path (other organs = spleen, heart, genitals) [50]
Among the routes by which lung toxicity can occur, some investigations favor the hypothesis that the surface area may be the most appropriate dose indicator for TiO2 NPs [11]. The ultrafine TiO2 NPs have high quality or low volatility that can damage the lungs at low dose [54] as compared to fine PSLT particles that increase inflammatory reaction and lung retention, e.g., nano-PSLT particles [55, 56]. After treating the rats with the TiO2 NPs, a high level of inflammatory reactions were observed due to increased NP surface size as compared to particles having a large surface area. According to some researches, TiO2 NPs cause a more significant pulmonary inflammation than large particles of TiO2, when a similar mass dose is introduced [57, 58]. However, when the dose is normalized in the surface area, the lungs’ response is the same because of nanosized and fine TiO2 particles. Therefore, in the study of lung toxicity, particles of different sizes of the same chemistry proved to be better.
Moreover, other investigations suggest that inflammatory reactions are likely to be more severe with the large surface area of nanoparticles [7, 59]. However, many studies proved that TiO2 NPs has more side effects [42]. These nanoparticles may cause immunological and pathological changes after accumulation [60] and can induce hepatic injury by altering biochemical parameters of serum (ALT, LDH, and BUN) depending on the amount and size of particles [53].
TiO2 NPs also induce brain injury because of their high vulnerability to oxidative stress [61,62,63]. Olfactory nerve and hippocampal neurons are considered the pathways for NPs when administered through nasal route under oxidative stress that decreases mice’s spatial recognition memory ability [64]. Moreover, TiO2 NPs can also decrease special recognition memory by disturbing the homeostasis of neurotransmitters, trace elements, and enzymes [19]. Many studies revealed a toxic effect depending on the duration of exposure and the dose of NPs [65,66,67,68]. These NPs elicit apoptosis and may accumulate in the brain, causing the increment in malondialdehyde (MDA), superoxide, water, 8-hydroxy-2′-deoxyguanosine, and carbonyl protein [69]. In addition, changes in the expression of associated genes also occur [70] that stimulate brain microglia to disturb mitochondrial energy with the production of ROS [71]. These particles also have a toxic effect on the glial cells by inducing morphological changes, with an increase in mitochondrial membrane potential (MMP) [72].
6 Oxidative Stress Induced by TiO2 NPs
Oxidative stress is considered a key mechanism for harmful biological effects by NPs [73]. This mechanism is confirmed by the increase in ROS production, oxidative products, and depletion of cellular antioxidants [74]. Oxidative stress is generally considered to be one of the major mechanisms of TiO2 NPs [73] which is associated with hydroxyl (OH) formation, DNA damage [75], and a high level of glutathione and liver’s malondialdehyde [76].
TiO2 mediates oxidative stress to produce different amounts of hydroxyl radicals with or without UV light exposure [77]. These hydroxyl radicals are the major destructive species that enhance DNA damage [75]. After initial exposure to ultraviolet light, anatase TiO2 particle sizes decrease cell viability in rats, resulting in DNA strand breaks and oxidative damage to DNA [78]. This is an important discovery, showing for the first time that photo-activated TiO2 particles retain higher cytotoxic and genotoxic potential regardless of particle size when UV irradiation is stopped because ROS is also a vital signal regulator [78]. Exposure of NPs to cells can also affect the cellular signaling cascade that controls processes such as cell proliferation, inflammation, and cell death by increasing ROS formation [79]. ROS production depends on the activation of inflammatory cascades such as phosphorylation of the Extracellular Signaling-Regulated Kinase ERK1/2 (ERK1/2), Tumor Necrosis Factor alpha (TNFα), and macrophage production together. High level of TiO2 NP stress leads to cell damage associated with the moderation of oxidative stress and inflammatory signaling pathways [71, 79,80,81].
7 Cellular Uptake of TiO2 NPs
From a toxicological point of view, the main characteristics of TiO2 NPs are its surface area, size, chemical properties, solubility, crystallinity, and the accumulation of particles [82]. Cell uptake, subcellular localization, and toxicity depend on the nature of these nanoparticles [83]. There are two main methods for the absorption of NPs in cells: active absorption in endocytosis and passive absorption in free diffusion [84]. Inhalation of TiO2 NPs may stimulate alveolar macrophages to remove micrometer sized particles (3–6 μm), but not TiO2 nanoparticles as they have very less size (20 nm) [84]. Phagocytosis normally removes particles larger than 500 nm because they cannot absorb the small particles [85]. So, particles remain in the tissue and cause constant stress on other tissues to endocytosis [86]. Results indicate that uptake of 50 nm nano-TiO2 by endocytosis with alveolar A549 epithelial cells are limited to aggregate particles [86]. Rothen-Rutishauser et al. [87] used an in vitro model of the airway walls in which membrane-bound aggregates (> 200 nm) and unbound aggregates were observed in the cytoplasm. They found highly aggregated NPs in both the late and early endosomes. TiO2 NP aggregates of less than 200 nm were able to penetrate red blood cells, but large particles were attached to the cell surface only [87].
8 Genotoxicity
Numerous in vitro and in vivo studies have been conducted to explore the TiO2 NP genotoxic effects including DNA damage, inflammatory cytokines, gene mutations, DNA deletions, and micronuclei formation that is indicative of chromosomal aberrations in different cell lines [88, 89]. The genotoxic effects depended upon TiO2 NP size and form [11] (Table 2).
9 Future Prospective
Nanotechnology develops the latest products and materials with improved properties. Existing data on nanoparticles show that these NPs spread throughout the body and accumulate in many organs by avoiding numerous protective barriers. Different forms of TiO2 NPs work differently due to the flexibility in shape, particle size, bioavailability, crystal structure, and UV-induced photocatalytic activity. So, it is suggested that TiO2 nanoparticles should be used with great care, especially in foods and cosmetics. Nanoscale TiO2 concentration must be declared in these products, so consumers are aware of the side effects of these products because these particles have a detrimental effect at the cellular, intracellular, and protein levels. Therefore, specific measures need to be taken to avoid the risk of disease for researchers, students, and workers during the manufacturing of these nanoparticles.
Abbreviations
- TiO2 :
-
Titanium dioxide
- NPs:
-
Nanoparticles
- PSLT:
-
Poorly soluble low-toxicity
- ALT:
-
Alanine aminotransferase
- LDH:
-
Lactic acid dehydrogenase
- BUN:
-
Blood urea nitrogen
References
Khan I, Saeed K, & Khan I. (2019). Nanoparticles: Properties, applications and toxicities. Arabian Journal of Chemistry, 12(7), 908–931.
Salata, O. V. (2004). Applications of nanoparticles in biology and medicine. Journal of Nanobiotechnology, 2(1), 3.
Saehana, S., Prasetyowati, R., Hidayat, M. I., Arifin, P., & Khairurrijal, A. (2011). Efficiency improvement in TiO2-particle based solar cells after deposition of metal in spaces between particles. International Journal of Basic and Applied Sciences, 12, 15.
Falck, G., Lindberg, H., Suhonen, S., Vippola, M., Vanhala, E., Catalan, J., Savolainen, K., & Norppa, H. (2009). Genotoxic effects of nanosized and fine TiO2. Human & Experimental Toxicology, 28(6-7), 339–352.
Manke A, Wang L, & Rojanasakul Y. (2013). Mechanisms of Nanoparticle-Induced Oxidative Stress and Toxicity. BioMed Research International, 2013, 942916.
Iavicoli, I., Leso, V., & Bergamaschi, A. (2012). Toxicological effects of titanium dioxide nanoparticles: a review of in vivo studies. Journal of Nanomaterials, 2012, 5.
Sayes, C. M., Wahi, R., Kurian, P. A., Liu, Y., West, J. L., Ausman, K. D., Warheit, D. B., & Colvin, V. L. (2006). Correlating nanoscale titania structure with toxicity: a cytotoxicity and inflammatory response study with human dermal fibroblasts and human lung epithelial cells. Toxicological Sciences, 92(1), 174–185.
Dunford, R., Salinaro, A., Cai, L., Serpone, N., Horikoshi, S., Hidaka, H., & Knowland, J. (1997). Chemical oxidation and DNA damage zcatalyzed by inorganic sunscreen ingredients. FEBS Letters, 418(1-2), 87–90.
Koedrith, P., Kim, Y. J., Kim, Y., Kang, J.-H., & Seo, Y. R. (2018). Intrinsic toxicity of stable nanosized titanium dioxide using polyacrylate in human keratinocytes. Molecular & Cellular Toxicology, 14(3), 273–282.
Sungur Ş. (2020). Titanium Dioxide Nanoparticles. In: Handbook of Nanomaterials and Nanocomposites for Energy and Environmental Applications, pp 1–18.
Shi, H., Magaye, R., Castranova, V., & Zhao, J. (2013). Titanium dioxide nanoparticles: a review of current toxicological data. Particle and Fibre Toxicology, 10(1), 15.
Ali, I., Suhail, M., Alothman, Z. A., & Alwarthan, A. (2018). Recent advances in syntheses, properties and applications of TiO 2 nanostructures. RSC Advances, 8(53), 30125–30147.
Stepanov AL, Xiao X, & Ren F. (2013). Implantation of titanium dioxide with transition metal ions. In K. J. Prafulla (Ed.), Titanium Dioxide: Applications, Synthesis and Toxicity (pp. 59–83). New York: Nova Science Publishers, Inc.
Toyooka, T., Amano, T., & Ibuki, Y. (2012). Titanium dioxide particles phosphorylate histone H2AX independent of ROS production. Mutation Research, Genetic Toxicology and Environmental Mutagenesis, 742(1-2), 84–91.
Magdolenova, Z., Bilaničová, D., Pojana, G., Fjellsbø, L. M., Hudecova, A., Hasplova, K., Marcomini, A., & Dusinska, M. (2012). Impact of agglomeration and different dispersions of titanium dioxide nanoparticles on the human related in vitro cytotoxicity and genotoxicity. Journal of Environmental Monitoring, 14(2), 455–464.
Hsiao, I.-L., & Huang, Y.-J. (2011). Effects of various physicochemical characteristics on the toxicities of ZnO and TiO2 nanoparticles toward human lung epithelial cells. ScTEn, 409(7), 1219–1228.
Robichaud CO, Uyar AE, Darby MR, Zucker LG, & Wiesner MR. (2009). Estimates of Upper Bounds and Trends in Nano-TiO2 Production As a Basis for Exposure Assessment. Environmental Science & Technology, 43(12), 4227–4233.
Landsiedel, R., Ma-Hock, L., Kroll, A., Hahn, D., Schnekenburger, J., Wiench, K., & Wohlleben, W. (2010). Testing metal-oxide nanomaterials for human safety. Advanced Materials, 22(24), 2601–2627.
Zhang, R., Bai, Y., Zhang, B., Chen, L., & Yan, B. (2012). The potential health risk of titania nanoparticles. Journal of Hazardous Materials, 211, 404–413.
Mowat, F., Tsuji, J.. Nanotechnology and the water market: applications and health effects. In: Technical Proceedings of the 2006 NSTI Nanotechnology Conference and Trade Show, US, 2006. pp 416-419.
Lv, J., Christie, P., & Zhang, S. (2019). Uptake, translocation, and transformation of metal-based nanoparticles in plants: recent advances and methodological challenges. Environmental Science: Nano, 6(1), 41–59.
Tang, T., Zhang, Z., & Zhu, X. (2019). Toxic effects of TiO2 NPs on zebrafish. International Journal of Environmental Research and Public Health, 16(4), 523.
Kiser, M., Westerhoff, P., Benn, T., Wang, Y., Perez-Rivera, J., & Hristovski, K. (2009). Titanium nanomaterial removal and release from wastewater treatment plants. Environmental Science & Technology, 43(17), 6757–6763.
Baranowska-Wójcik, E., Szwajgier, D., Oleszczuk, P., & Winiarska-Mieczan A. (2020). Effects of Titanium Dioxide Nanoparticles Exposure on Human Health—a Review. Biological Trace Element Research, 193(1), 118–129.
Hou, J., Wang, L., Wang, C., Zhang S., Liu, H., Li, S., & Wang, X. (2019). Toxicity and mechanisms of action of titanium dioxide nanoparticles in living organisms. Journal of Environmental Sciences, 75, 40–53.
Liu, R., Zhang, X., Pu, Y., Yin, L., Li, Y., Zhang, X., Liang, G., Li, X., & Zhang, J. (2010). Small-sized titanium dioxide nanoparticles mediate immune toxicity in rat pulmonary alveolar macrophages in vivo. Journal of Nanoscience and Nanotechnology, 10(8), 5161–5169.
Acar, M., Bulut, Z., Ateş, A., Nami, B., Koçak, N., & Yıldız, B. (2015). Titanium dioxide nanoparticles induce cytotoxicity and reduce mitotic index in human amniotic fluid-derived cells. Human & Experimental Toxicology, 34(1), 74–82.
Coccini, T., Grandi, S., Lonati, D., Locatelli, C., & De Simone, U. (2015). Comparative cellular toxicity of titanium dioxide nanoparticles on human astrocyte and neuronal cells after acute and prolonged exposure. Neurotoxicology, 48, 77–89.
Lammel, T., Mackevica, A., Johansson, B. R., & Sturve, J. (2019). Endocytosis, intracellular fate, accumulation, and agglomeration of titanium dioxide (TiO 2) nanoparticles in the rainbow trout liver cell line RTL-W1. Environmental Science and Pollution Research, 26(15), 15354–15372.
Biola-Clier, M., Gaillard, J.-C., Rabilloud, T., Armengaud, J., & Carriere, M. (2020). Titanium dioxide nanoparticles alter the cellular phosphoproteome in A549 cells. Nanomaterials, 10(2), 185.
Song, Y., Li, X., & Du, X. (2009). Exposure to nanoparticles is related to pleural effusion, pulmonary fibrosis and granuloma. The European Respiratory Journal, 34(3), 559–567.
Lu, N., Zhu, Z., Zhao, X., Tao, R., Yang, X., & Gao, Z. (2008). Nano titanium dioxide photocatalytic protein tyrosine nitration: a potential hazard of TiO2 on skin. Biochemical and Biophysical Research Communications, 370(4), 675–680.
Mercer, R. R., Scabilloni, J., Wang, L., Kisin, E., Murray, A. R., Schwegler-Berry, D., Shvedova, A. A., & Castranova, V. (2008). Alteration of deposition pattern and pulmonary response as a result of improved dispersion of aspirated single-walled carbon nanotubes in a mouse model. American Journal of Physiology. Lung Cellular and Molecular Physiology, 294(1), L87–L97.
Hamilton Jr., R. F., Buford, M. C., Wood, M. B., Arnone, B., Morandi, M., & Holian, A. (2007). Engineered carbon nanoparticles alter macrophage immune function and initiate airway hyper-responsiveness in the BALB/c mouse model. Nanotoxicology, 1(2), 104–117.
Boffetta, P., Gaborieau, V., Nadon, L., Parent, M.-E., Weiderpass, E., & Siemiatycki, J. (2001). Exposure to titanium dioxide and risk of lung cancer in a population-based study from Montreal. Scandinavian Journal of Work, Environment & Health, 27(4), 227–232.
Behnam, M. A., Emami, F., Sobhani, Z., & Dehghanian, A. R. (2018). The application of titanium dioxide (TiO2) nanoparticles in the photo-thermal therapy of melanoma cancer model. Iranian Journal of Basic Medical Sciences, 21(11), 1133.
Fujiwara, R., Luo, Y., Sasaki, T., Fujii, K., Ohmori, H., & Kuniyasu, H. (2015). Cancer therapeutic effects of titanium dioxide nanoparticles are associated with oxidative stress and cytokine induction. Pathobiology, 82(6), 243–251.
Hussain, S., Vanoirbeek, J. A., Luyts, K., De Vooght, V., Verbeken, E., Thomassen, L. C., Martens, J. A., Dinsdale, D., Boland, S., & Marano, F. (2011). Lung exposure to nanoparticles modulates an asthmatic response in a mouse model. The European Respiratory Journal, 37(2), 299–309.
Leppänen, M., Korpi, A., Miettinen, M., Leskinen, J., Torvela, T., Rossi, E. M., Vanhala, E., Wolff, H., Alenius, H., & Kosma, V.-M. (2011). Nanosized TiO 2 caused minor airflow limitation in the murine airways. Archives of Toxicology, 85(7), 827–839.
Yamashita, K., Yoshioka, Y., Higashisaka, K., Mimura, K., Morishita, Y., Nozaki, M., Yoshida, T., Ogura, T., Nabeshi, H., & Nagano, K. (2011). Silica and titanium dioxide nanoparticles cause pregnancy complications in mice. Nature Nanotechnology, 6(5), 321–328.
Yanagisawa, R., Takano, H., K-i, I., Koike, E., Kamachi, T., Sadakane, K., & Ichinose, T. (2009). Titanium dioxide nanoparticles aggravate atopic dermatitis-like skin lesions in NC/Nga mice. Experimental Biology and Medicine, 234(3), 314–322.
Nemmar, A., Melghit, K., Al-Salam, S., Zia, S., Dhanasekaran, S., Attoub, S., Al-Amri, I., & Ali, B. H. (2011). Acute respiratory and systemic toxicity of pulmonary exposure to rutile Fe-doped TiO2 nanorods. Toxicology, 279(1-3), 167–175.
Jia, X., Wang, S., Zhou, L., & Sun, L. (2017). The potential liver, brain, and embryo toxicity of titanium dioxide nanoparticles on mice. Nanoscale Research Letters, 12(1), 478.
Tang, M., Zhang, T., Xue, Y., Wang, S., Huang, M., Yang, Y., Lu, M., Lei, H., Kong, L., & Wang, Y. (2011). Metabonomic studies of biochemical changes in the serum of rats by intratracheally instilled TiO2 nanoparticles. Journal of Nanoscience and Nanotechnology, 11(4), 3065–3074.
Wang, J.-X., Fan, Y.-B., Gao, Y., Hu, Q.-H., & Wang, T.-C. (2009). TiO2 nanoparticles translocation and potential toxicological effect in rats after intraarticular injection. Biomaterials, 30(27), 4590–4600.
Takeda, K., K-i, S., Ishihara, A., Kubo-Irie, M., Fujimoto, R., Tabata, M., Oshio, S., Nihei, Y., Ihara, T., & Sugamata, M. (2009). Nanoparticles transferred from pregnant mice to their offspring can damage the genital and cranial nerve systems. Journal of Health Science, 55(1), 95–102.
Bahadar, H., Maqbool, F., Niaz, K., & Abdollahi, M. (2016). Toxicity of nanoparticles and an overview of current experimental models. Iranian Biomedical Journal, 20(1), 1.
Song, B., Zhang, Y., Liu, J., Feng, X., Zhou, T., & Shao, L. (2016). Is neurotoxicity of metallic nanoparticles the cascades of oxidative stress? Nanoscale Research Letters, 11(1), 291.
Faddah, L., Baky, N. A. A., Al-Rasheed, N. M., & Al-Rasheed, N. M. (2013). Biochemical responses of nanosize titanium dioxide in the heart of rats following administration of idepenone and quercetin LM Faddah*, Nayira A. Abdel Baky, Nouf M. Al-Rasheed and Nawal M. Al-Rasheed. African Journal of Pharmacy and Pharmacology, 7(38), 2639–2651.
Hagens, W. I., Oomen, A. G., de Jong, W. H., Cassee, F. R., & Sips, A. J. (2007). What do we (need to) know about the kinetic properties of nanoparticles in the body? Regulatory Toxicology and Pharmacology, 49(3), 217–229.
McClements, D. J., Xiao, H., & Demokritou, P. (2017). Physicochemical and colloidal aspects of food matrix effects on gastrointestinal fate of ingested inorganic nanoparticles. Advances in Colloid and Interface Science, 246, 165–180.
Patri, A., Umbreit, T., Zheng, J., Nagashima, K., Goering, P., Francke-Carroll, S., Gordon, E., Weaver, J., Miller, T., & Sadrieh, N. (2009). Energy dispersive X-ray analysis of titanium dioxide nanoparticle distribution after intravenous and subcutaneous injection in mice. Journal of Applied Toxicology, 29(8), 662–672.
Wang, J., Zhou, G., Chen, C., Yu, H., Wang, T., Ma, Y., Jia, G., Gao, Y., Li, B., & Sun, J. (2007). Acute toxicity and biodistribution of different sized titanium dioxide particles in mice after oral administration. Toxicology Letters, 168(2), 176–185.
Wang, Y., Cui, H., Zhou, J., Li, F., Wang, J., Chen, M., & Liu, Q. (2015). Cytotoxicity, DNA damage, and apoptosis induced by titanium dioxide nanoparticles in human non-small cell lung cancer A549 cells. Environmental Science and Pollution Research, 22(7), 5519–5530.
Schulte, P. A., Kuempel, E. D., & Drew, N. M. (2018). Characterizing risk assessments for the development of occupational exposure limits for engineered nanomaterials. Regulatory Toxicology and Pharmacology, 95, 207–219.
Tran, C., Buchanan, D., Cullen, R., Searl, A., Jones, A., & Donaldson, K. (2000). Inhalation of poorly soluble particles II. Influence of particle surface area on inflammation and clearance. Inhalation Toxicology, 12(12), 1113–1126.
Sager, T. M., Kommineni, C., & Castranova, V. (2008). Pulmonary response to intratracheal instillation of ultrafine versus fine titanium dioxide: role of particle surface area. Particle and Fibre Toxicology, 5(1), 17.
Oberdörster, G., Oberdörster, E., & Oberdörster, J. (2005). Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environmental Health Perspectives, 113(7), 823–839.
Warheit, D. B., Webb, T. R., Reed, K. L., Frerichs, S., & Sayes, C. M. (2007). Pulmonary toxicity study in rats with three forms of ultrafine-TiO2 particles: differential responses related to surface properties. Toxicology, 230(1), 90–104.
Li, N., Duan, Y., Hong, M., Zheng, L., Fei, M., Zhao, X., Wang, J., Cui, Y., Liu, H., & Cai, J. (2010). Spleen injury and apoptotic pathway in mice caused by titanium dioxide nanoparticules. Toxicology Letters, 195(2-3), 161–168.
Kreyling, W. G., Holzwarth, U., Haberl, N., Kozempel, J., Hirn, S., Wenk, A., Schleh, C., Schäffler, M., Lipka, J., & Semmler-Behnke, M. (2017). Quantitative biokinetics of titanium dioxide nanoparticles after intravenous injection in rats: part 1. Nanotoxicology, 11(4), 434–442.
Kreyling, W. G., Holzwarth, U., Schleh, C., Kozempel, J., Wenk, A., Haberl, N., Hirn, S., Schäffler, M., Lipka, J., & Semmler-Behnke, M. (2017). Quantitative biokinetics of titanium dioxide nanoparticles after oral application in rats: part 2. Nanotoxicology, 11(4), 443–453.
Kreyling, W. G., Holzwarth, U., Haberl, N., Kozempel, J., Wenk, A., Hirn, S., Schleh, C., Schäffler, M., Lipka, J., & Semmler-Behnke, M. (2017). Quantitative biokinetics of titanium dioxide nanoparticles after intratracheal instillation in rats: Part 3. Nanotoxicology, 11(4), 454–464.
Wang, J., Chen, C., Liu, Y., Jiao, F., Li, W., Lao, F., Li, Y., Li, B., Ge, C., & Zhou, G. (2008). Potential neurological lesion after nasal instillation of TiO2 nanoparticles in the anatase and rutile crystal phases. Toxicology Letters, 183(1-3), 72–80.
Czajka, M., Sawicki, K., Sikorska, K., Popek, S., Kruszewski, M., & Kapka-Skrzypczak, L. (2015). Toxicity of titanium dioxide nanoparticles in central nervous system. Toxicology In Vitro, 29(5), 1042–1052.
Grissa, I., Guezguez, S., Ezzi, L., Chakroun, S., Sallem, A., Kerkeni, E., Elghoul, J., El Mir, L., Mehdi, M., & Ben Cheikh, H. (2016). The effect of titanium dioxide nanoparticles on neuroinflammation response in rat brain. Environmental Science and Pollution Research, 23(20), 20205–20213.
Valentini, X., Deneufbourg, P., Paci, P., Rugira, P., Laurent, S., Frau, A., Stanicki, D., Ris, L., & Nonclercq, D. (2018). Morphological alterations induced by the exposure to TiO2 nanoparticles in primary cortical neuron cultures and in the brain of rats. Toxicology Reports, 5, 878–889.
Li, X., Xu, S., Zhang, Z., & Schluesener, H. J. (2009). Apoptosis induced by titanium dioxide nanoparticles in cultured murine microglia N9 cells. Chinese Science Bulletin, 54(20), 3830–3836.
Ze, Y., Hu, R., Wang, X., Sang, X., Ze, X., Li, B., Su, J., Wang, Y., Guan, N., & Zhao, X. (2014). Neurotoxicity and gene-expressed profile in brain-injured mice caused by exposure to titanium dioxide nanoparticles. Journal of Biomedical Materials Research Part A, 102(2), 470–478.
Long, T. C., Saleh, N., Tilton, R. D., Lowry, G. V., & Veronesi, B. (2006). Titanium dioxide (P25) produces reactive oxygen species in immortalized brain microglia (BV2): implications for nanoparticle neurotoxicity. Environmental Science & Technology, 40(14), 4346–4352.
Gurr, J.-R., Wang, A. S., Chen, C.-H., & Jan, K.-Y. (2005). Ultrafine titanium dioxide particles in the absence of photoactivation can induce oxidative damage to human bronchial epithelial cells. Toxicology, 213(1-2), 66–73.
Huerta-García, E., Pérez-Arizti, J. A., Márquez-Ramírez, S. G., Delgado-Buenrostro, N. L., Chirino, Y. I., Iglesias, G. G., & López-Marure, R. (2014). Titanium dioxide nanoparticles induce strong oxidative stress and mitochondrial damage in glial cells. Free Radical Biology and Medicine, 73, 84–94.
Kohen, R., & Nyska, A. (2002). Invited review: oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicologic Pathology, 30(6), 620–650.
Hou, J., Wang, L., Wang, C., Zhang, S., Liu, H., Li, S., & Wang, X. (2019). Toxicity and mechanisms of action of titanium dioxide nanoparticles in living organisms. Journal of Environmental Sciences, 75, 40–53.
Reeves, J. F., Davies, S. J., Dodd, N. J., & Jha, A. N. (2008). Hydroxyl radicals (OH) are associated with titanium dioxide (TiO2) nanoparticle-induced cytotoxicity and oxidative DNA damage in fish cells. Mutation Research, Fundamental and Molecular Mechanisms of Mutagenesis, 640(1-2), 113–122.
Rajapakse, K., Drobne, D., Valant, J., Vodovnik, M., Levart, A., & Marinsek-Logar, R. (2012). Acclimation of Tetrahymena thermophila to bulk and nano-TiO2 particles by changes in membrane fatty acids saturation. Journal of Hazardous Materials, 221, 199–205.
Uchino, T., Tokunaga, H., Ando, M., & Utsumi, H. (2002). Quantitative determination of OH radical generation and its cytotoxicity induced by TiO2–UVA treatment. Toxicology In Vitro, 16(5), 629–635.
Petković, J., Küzma, T., Rade, K., Novak, S., & Filipič, M. (2011). Pre-irradiation of anatase TiO2 particles with UV enhances their cytotoxic and genotoxic potential in human hepatoma HepG2 cells. Journal of Hazardous Materials, 196, 145–152.
Barthel, A., & Klotz, L.-O. (2005). Phosphoinositide 3-kinase signaling in the cellular response to oxidative stress. Biological Chemistry, 386(3), 207–216.
Petković, J., Žegura, B., Stevanović, M., Drnovšek, N., Uskoković, D., Novak, S., & Filipič, M. (2011). DNA damage and alterations in expression of DNA damage responsive genes induced by TiO2 nanoparticles in human hepatoma HepG2 cells. Nanotoxicology, 5(3), 341–353.
Kang, J. L., Moon, C., Lee, H. S., Lee, H. W., Park, E.-M., Kim, H. S., & Castranova, V. (2008). Comparison of the biological activity between ultrafine and fine titanium dioxide particles in RAW 264.7 cells associated with oxidative stress. Journal of Toxicology and Environmental Health. Part A, 71(8), 478–485.
Walters, C., Pool, E., & Somerset, V. (2016). Nanotoxicology: A Review. In S. Soloneski & M. L. Larramendy (Eds.), Toxicology - New Aspects to This Scientific Conundrum (pp. 45–63). Rijeka: IntechOpen.
Xia, T., Kovochich, M., Brant, J., Hotze, M., Sempf, J., Oberley, T., Sioutas, C., Yeh, J. I., Wiesner, M. R., & Nel, A. E. (2006). Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm. Nano Letters, 6(8), 1794–1807.
Geiser, M., Casaulta, M., Kupferschmid, B., Schulz, H., Semmler-Behnke, M., & Kreyling, W. (2008). The role of macrophages in the clearance of inhaled ultrafine titanium dioxide particles. American Journal of Respiratory Cell and Molecular Biology, 38(3), 371–376.
Aderem, A., & Underhill, D. M. (1999). Mechanisms of phagocytosis in macrophages. Annual Review of Immunology, 17(1), 593–623.
Liu, X., Sui, B., & Sun, J. (2017). Size-and shape-dependent effects of titanium dioxide nanoparticles on the permeabilization of the blood–brain barrier. Journal of Materials Chemistry B, 5(48), 9558–9570.
Rothen-Rutishauser, B. M., Schürch, S., Haenni, B., Kapp, N., & Gehr, P. (2006). Interaction of fine particles and nanoparticles with red blood cells visualized with advanced microscopic techniques. Environmental Science & Technology, 40(14), 4353–4359.
Shukla, R. K., Sharma, V., Pandey, A. K., Singh, S., Sultana, S., & Dhawan, A. (2011). ROS-mediated genotoxicity induced by titanium dioxide nanoparticles in human epidermal cells. Toxicology In Vitro, 25(1), 231–241.
Charles, S., Jomini, S., Fessard, V., Bigorgne-Vizade, E., Rousselle, C., & Michel, C. (2018). Assessment of the in vitro genotoxicity of TiO2 nanoparticles in a regulatory context. Nanotoxicology, 12(4), 357–374.
Rizk, M. Z., Ali, S. A., Hamed, M. A., El-Rigal, N. S., Aly, H. F., & Salah, H. H. (2017). Toxicity of titanium dioxide nanoparticles: effect of dose and time on biochemical disturbance, oxidative stress and genotoxicity in mice. Biomedicine & Pharmacotherapy, 90, 466–472.
Valentini, X., Rugira, P., Frau, A., Tagliatti, V., Conotte, R., Laurent, S., Colet J-M, & Nonclercq, D. (2019). Hepatic and Renal Toxicity Induced by TiO2 Nanoparticles in Rats: A Morphological and Metabonomic Study. Journal of Toxicology, 2019, 5767012.
Louro, H., Tavares, A., Vital, N., Costa, P. M., Alverca, E., Zwart, E., De Jong, W. H., Fessard, V., Lavinha, J., & Silva, M. J. (2014). Integrated approach to the in vivo genotoxic effects of a titanium dioxide nanomaterial using LacZ plasmid-based transgenic mice. Environmental and Molecular Mutagenesis, 55(6), 500–509.
Ma, Y., Guo, Y., Wu, S., Lv, Z., Zhang, Q., & Ke, Y. (2017). Titanium dioxide nanoparticles induce size-dependent cytotoxicity and genomic DNA hypomethylation in human respiratory cells. RSC Advances, 7(38), 23560–23572.
Mohamed, H. R. H., & Hussien, N. A. (2016). Genotoxicity Studies of Titanium Dioxide Nanoparticles (TiO2NPs) in the Brain of Mice. Scientifica, 2016, 6710840.
Lindberg, H. K., Falck, G. C.-M., Catalán, J., Koivisto, A. J., Suhonen, S., Järventaus, H., Rossi, E. M., Nykäsenoja, H., Peltonen, Y., & Moreno, C. (2012). Genotoxicity of inhaled nanosized TiO2 in mice. Mutation Research, Genetic Toxicology and Environmental Mutagenesis, 745(1-2), 58–64.
Patel, S., Patel, P., & Bakshi, S. R. (2017). Titanium dioxide nanoparticles: an in vitro study of DNA binding, chromosome aberration assay, and comet assay. Cytotechnology, 69(2), 245–263.
Pakrashi, S., Jain, N., Dalai, S., Jayakumar, J., Chandrasekaran, P. T., Raichur, A. M., Chandrasekaran, N., & Mukherjee, A. (2014). In vivo genotoxicity assessment of titanium dioxide nanoparticles by Allium cepa root tip assay at high exposure concentrations. PLoS One, 9(2), e87789.
Li, Y., Yan, J., Ding, W., Chen, Y., Pack, L. M., & Chen, T. (2016). Genotoxicity and gene expression analyses of liver and lung tissues of mice treated with titanium dioxide nanoparticles. Mutagenesis, 32(1), 33–46.
Suzuki, T., Miura, N., Hojo, R., Yanagiba, Y., Suda, M., Hasegawa, T., Miyagawa, M., & Wang, R.-S. (2016). Genotoxicity assessment of intravenously injected titanium dioxide nanoparticles in gpt delta transgenic mice. Mutation Research, Genetic Toxicology and Environmental Mutagenesis, 802, 30–37.
Carmona, E. R., Escobar, B., Vales, G., & Marcos, R. (2015). Genotoxic testing of titanium dioxide anatase nanoparticles using the wing-spot test and the comet assay in Drosophila. Mutation Research, Genetic Toxicology and Environmental Mutagenesis, 778, 12–21.
de Melo, R. É., de Rezende, A. A. A., de Oliveira, P. F., Nicolella, H. D., Tavares, D. C., Silva, A. C. A., Dantas, N. O., & Spanó, M. A. (2016). Evaluation of titanium dioxide nanocrystal-induced genotoxicity by the cytokinesis-block micronucleus assay and the Drosophila wing spot test. Food and Chemical Toxicology, 96, 309–319.
Xu, J., Shi, H., Ruth, M., Yu, H., Lazar, L., Zou, B., Yang, C., Wu, A., & Zhao, J. (2013). Acute toxicity of intravenously administered titanium dioxide nanoparticles in mice. PLoS One, 8(8), e70618.
Rahman, L., Wu, D., Johnston, M., Williams, A., & Halappanavar, S. (2016). Toxicogenomics analysis of mouse lung responses following exposure to titanium dioxide nanomaterials reveal their disease potential at high doses. Mutagenesis, 32(1), 59–76.
Chen, Z., Wang, Y., Ba, T., Li, Y., Pu, J., Chen, T., Song, Y., Gu, Y., Qian, Q., & Yang, J. (2014). Genotoxic evaluation of titanium dioxide nanoparticles in vivo and in vitro. Toxicology Letters, 226(3), 314–319.
Asare, N., Duale, N., Slagsvold, H. H., Lindeman, B., Olsen, A. K., Gromadzka-Ostrowska, J., Meczynska-Wielgosz, S., Kruszewski, M., Brunborg, G., & Instanes, C. (2016). Genotoxicity and gene expression modulation of silver and titanium dioxide nanoparticles in mice. Nanotoxicology, 10(3), 312–321.
Valentini, X., Absil, L., Laurent, G., Robbe, A., Laurent, S., Muller, R., Legrand, A., & Nonclercq, D. (2017). Toxicity of TiO 2 nanoparticles on the NRK52E renal cell line. Molecular & Cellular Toxicology, 13(4), 419–431.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Research Involving Human Participants and/or Animals
None.
Informed Consent
None.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shabbir, S., Kulyar, M.FeA., Bhutta, Z.A. et al. Toxicological Consequences of Titanium Dioxide Nanoparticles (TiO2NPs) and Their Jeopardy to Human Population. BioNanoSci. 11, 621–632 (2021). https://doi.org/10.1007/s12668-021-00836-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-021-00836-3