Abstract
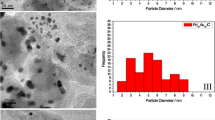
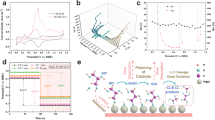
In this work, PtSn binary electrodeposits were prepared in three compositions and submitted to successive voltammetric cycles in presence of glycerol (1.0 mol L−1) in acidic media. Catalysts were characterized by energy dispersive X-ray analysis and X-ray photoelectron spectroscopy before and after the cycles being performed, in order to check eventual changes in their compositions during the process. Spectroscopic results show that surface compositions are sensibly richer in Sn than their bulk counterparts. Overall, PtSn catalysts show a poor initial catalytic activity toward glycerol electrooxidation. However, as the cycles succeed, the voltammetric responses increasingly resemble that of Pt, while the oxidation currents increase. Results are rationalized in terms of a continuous enrichment of the surface by Pt at the expenses of a loss of Sn. Moreover, when the electrochemical surface area (ECSA) is estimated by stripping of CO, it becomes evident that electrooxidation currents remain growing, even when the ECSA is decreased, which makes the gain in catalytic activity particularly relevant. Ultimately, from a broader perspective, our results suggest that catalytic surfaces with tunable features (such as surface composition and catalytic response) can be obtained by the application of easily executable electrochemical protocols.

ᅟ







Similar content being viewed by others
References
C. A. G. Quispe, C. J. R. Coronado, J. A. Carvalho Jr., Glycerol: production, consumption, prices, characterization and new trends in combustion. Renew. Sust. Energ. Rev. 27, 475 (2013)
P. S. Fernández, M. E. Martins, G. A. Camara, New insights about the electro-oxidation of glycerol on platinum nanoparticles supported on multi-walled carbon nanotubes. Electrochim. Acta 66, 180 (2012)
J. F. Gomes, C. A. Martins, M. J. Giz, G. Tremiliosi-Filho, G. A. Camara, Insights into the adsorption and electro-oxidation of glycerol: self-inhibition and concentration effects. J. Catal. 301, 154 (2013)
L. Roquet, E. M. Belgsir, J.-M. Léger, C. Lamy, Kinetics and mechanisms of the electrocatalytic oxidation of glycerol as investigated by chromatographic analysis of the reaction products: potential and pH effects. Electrochim. Acta 39, 2387 (1994)
P. S. Fernández, C. A. Martins, M. E. Martins, G. A. Camara, Electrooxidation of glycerol on platinum nanoparticles: deciphering how the position of each carbon affects the oxidation pathways. Electrochim. Acta 112, 686 (2013)
C. A. Martins, P. S. Fernández, H. E. Troiani, M. E. Martins, G. A. Camara, Ethanol vs. glycerol: understanding the lack of correlation between the oxidation currents and the production of CO2 on Pt nanoparticles. J. Electroanal. Chem. 231, 717–718 (2014)
A. Kowal, M. Li, M. Shao, K. Sasaki, M. B. Vukmirovic, J. Zhang, N. S. Marinkovic, P. Liu, A. I. Frenkel, R. R. Adzic, Ternary Pt/Rh/SnO2 electrocatalysts for oxidizing ethanol to CO2. Nat. Mater. 8, 325 (2009)
M. Li, D. A. Cullen, K. Sasaki, N. S. Marinkovic, K. More, R. R. Adzic, Ternary electrocatalysts for oxidizing ethanol to carbon dioxide: making Ir capable of splitting C−C bond. J. Am. Chem. Soc. 135, 132 (2013)
C. Lamy, S. Rousseau, E. M. Belgsir, C. Coutanceau, J.-M. Léger, Recent progress in the direct ethanol fuel cell: development of new platinum-tin electrocatalysts. Electrochim. Acta 49, 3901 (2004)
F. Colmati, E. Antolini, E. R. Gonzalez, Effect of temperature on the mechanism of ethanol oxidation on carbon supported Pt, PtRu and Pt3Sn electrocatalysts. J. Power Sources 157, 98 (2006)
H. Wang, Z. Jusys, R. J. Behm, Ethanol electro-oxidation on carbon-supported Pt, PtRu and Pt3Sn catalysts: a quantitative DEMS study. J. Power Sources 154, 351 (2006)
S. Beyhan, C. Coutanceau, J.-M. Léger, T. W. Napporn, F. Kadrigan, Promising anode candidates for direct ethanol fuel cell: carbon-supported PtSn-based trimetallic catalysts prepared by Bonnemann method. Int. J. Hydrogen Energ. 38, 6830 (2013)
A. Falase, M. Main, K. Garcia, A. Serov, C. Lau, P. Atanassov, Electrooxidation of ethylene glycol and glycerol by platinum-based binary and ternary nano-structured catalysts. Electrochim. Acta 66, 295 (2012)
P. S. Fernández, M. E. Martins, C. A. Martins, G. A. Camara, The electro-oxidation of isotopically labeled glycerol on platinum: new information on C–C bond cleavage and CO2 production. Electrochem. Commun. 15, 14 (2012)
H. J. Kim, S. M. Choi, S. Green, G. A. Tompsett, S. H. Lee, G. W. Huber, W. B. Kim, Highly active and stable PtRuSn/C catalyst for electrooxidation on ethylene glycol and glycerol. Appl. Catal. B-Environ. 101, 366 (2011)
L. Zheng, L. Xiong, Q. Liu, K. Han, W. Liu, Y. Li, K. Tao, L. Niu, S. Yang, J. Xia, Enhanced electrocatalytic activity for the oxidation of liquid fuels on PtSn nanoparticles. Electrochim. Acta 56, 9860 (2011)
M. J. Giz, G. A. Camara, G. Maia, The etanol electrooxidation reaction at rough PtRu electrodeposits: a FTIR study. Electrochem. Commun. 11, 1586 (2009)
E. Casado-Rivera, D. J. Volpe, L. Alden, C. Lind, C. Downie, T. Vázquez-Alvarez, A. C. D. Angelo, F. J. DiSalvo, H. D. Abruña, Electrocatalytic activity of ordered intermetallic phases for fuel cell applications. J. Am. Chem. Soc. 126, 4043 (2004)
S. C. Zignani, V. Baglio, J. J. Linares, E. R. Gonzalez, A. S. Aricò, Endurance study of a solid polymer electrolyte direct ethanol fuel cell based on a Pt-Sn anode catalyst. Int. J. Hydrogen Energ. 38, 11576 (2013)
S. Stevanovic, D. Tripkovic, V. Tripkovic, D. Minic, A. Gavrilovic, A. Tripkovic, V. M. Jovanovic, Insight into the effect of Sn on CO and formic acid oxidation at PtSn catalysts. J. Phys. Chem. C 118, 278 (2014)
Acknowledgments
The authors thank CNPq (grant nos. 405695/2013-6 and 309176/2015-8), FUNDECT, CAPES, MINCyT, and FINEP for funding this study. G.A.B. Mello is indebted to CAPES for a doctorate fellowship. P.S. Fernández acknowledges CONICET for a post doctorate fellowship. M.E. Martins acknowledges Universidad Nacional de La Plata and CONICET (PIP 112-201101-00917).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mello, G.A.B., Fernández, P.S., Martins, M.E. et al. Glycerol Electrooxidation on Platinum-Tin Electrodeposited Films: Inducing Changes in Surface Composition by Cyclic Voltammetry. Electrocatalysis 8, 1–10 (2017). https://doi.org/10.1007/s12678-016-0332-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12678-016-0332-z