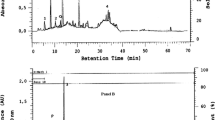
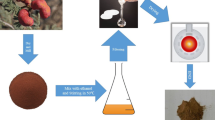
Abstract
The inhibitory performance of quince extract (QE) on the corrosion and electrochemical properties of low-carbon steel in 1 molar hydrochloric acid was studied by electrochemical methods and weight loss measurement. The chemical compounds contained in quince that offer substantial corrosion inhibition impacts (e.g., amino acids and flavonoids) were examined by Fourier-transform infrared spectroscopy and ultraviolet. Polarization tests and electrochemical impedance spectroscopy results showed that increasing the concentration of the extract in the range of 200–1200 ppm increased its inhibition efficiency. The highest inhibition efficiency of the extract based on polarization and EIS results was respectively 95% and 93.0%, both observed at a concentration of 1200 ppm. Based on the results, quince extract was considered as a mixed-type corrosion inhibitor. Scanning electron microscopy and atomic force microscopy of the surface of steel specimens immersed in 1 M HCl without and with 200–1200 ppm of the inhibitor showed the effectiveness of quince extract in preventing corrosion.
Graphical Abstract










Similar content being viewed by others
References
S. Umoren, I.B. Obot, Z. Gasem, N.A. Odewunmi, Experimental and theoretical studies of red apple fruit extract as green corrosion inhibitor for mild steel in HCl solution. J. Dispersion Sci. Technol. 36, 789 (2015)
P.E.A. Leena, Studies on methanolic extract of lepidagathis keralensis as green corrosion inhibitor for mild steel in 1M HCl. J. Electrochem. Sci. Technol. 10, 231 (2019)
D.B. Hmamou, R. Salghi, A. Zarrouk, H. Zarrok, R. Touzani, B. Hammouti, A. El Assyry, Investigation of corrosion inhibition of carbon steel in 0.5 M H2SO4 by new bipyrazole derivative using experimental and theoretical approaches. J. Environ. Chem. Eng. 3, 2031 (2015)
H. Wei, B. Heidarshenas, L. Zhou, G. Hussain, Q. Li, K. Ostrikov, Green inhibitors for steel corrosion in acidic environment: state of art. Mater. Today Sustain. 10, 100044 (2020)
A. Singh, V.K. Singh, M.A. Quraishi, Aqueous extract of Kalmegh (Andrographis paniculata) leaves as green inhibitor for mild steel in hydrochloric acid solution. Int. J. Corros. 2010, 1 (2010)
M. Nasibi, E. Rafiee, G. Rashed, H. Ashassi-Sorkhabi, M. Behpour, Corrosion inhibition of mild steel by Safflower (Carthamus tinctorius) extract: polarization, EIS, AFM, SEM, EDS, and artificial neural network modeling. J. Dispersion Sci. Technol. 34, 964 (2013)
E.A. Florez-Frias, V. Barba, R. Lopez-Sesenes, L.L. Landeros-Martínez, J.P.F.-D. los Ríos, M. Casales, J.G. Gonzalez-Rodriguez, M.I. Ojovan, Use of a metallic complex derived from Curcuma longa as green corrosion inhibitor for carbon cteel in sulfuric acid. Int. J. Corros. 2021, 1 (2021)
M. Ramezanzadeh, G. Bahlakeh, Z. Sanaei, B. Ramezanzadeh, Corrosion inhibition of mild steel in 1 M HCl solution by ethanolic extract of eco-friendly Mangifera indica (mango) leaves: electrochemical, molecular dynamics, Monte Carlo and ab initio study. Appl. Surf. Sci. 463, 1058 (2019)
M. Izadi, T. Shahrabi, B. Ramezanzadeh, Active corrosion protection performance of an epoxy coating applied on the mild steel modified with an eco-friendly sol-gel film impregnated with green corrosion inhibitor loaded nanocontainers. Appl. Surf. Sci. 440, 491 (2018)
B.E.A. Rani, B.B.J. Basu, Green inhibitors for corrosion protection of metals and alloys: an overview. Int. J. Corros. 2012, 1 (2012)
S. Marzorati, L. Verotta, S.P. Trasatti, Green corrosion inhibitors from natural sources and biomass wastes. Molecules. 24, (2018)
N.O. Eddy, E.E. Ebenso, Adsorption and inhibitive properties of ethanol extracts of Musa sapientum peels as a green corrosion inhibitor for mild steel in H2SO4. Afr. J. Pure Appl. Chem. 2, 046 (2008)
J.C. da Rocha, J.A. da Cunha Ponciano Gomes, E. D’Elia, Corrosion inhibition of carbon steel in hydrochloric acid solution by fruit peel aqueous extracts. Corros. Sci. 52, 2341 (2010)
V.V. Torres, R.S. Amado, C.F. de Sá, T.L. Fernandez, C.A.d.S. Riehl, A.G. Torres, E. D’Elia, Inhibitory action of aqueous coffee ground extracts on the corrosion of carbon steel in HCl solution. Corros. Sci. 53, 2385 (2011)
M.F. Heragh, H. Tavakoli, Synergetic effect of the combination of Prosopis farcta extract with sodium dodecyl sulfate on corrosion inhibition of St37 steel in 1M HCl medium. J. Mol. Struct. 1245, 131086 (2021)
S. Paul, I. Koley, Corrosion inhibition of carbon cteel in acidic environment by papaya seed as green inhibitor. J. Bio- Tribo-Corros. 2, 6 (2016)
Z. Sanaei, M. Ramezanzadeh, G. Bahlakeh, B. Ramezanzadeh, Use of Rosa canina fruit extract as a green corrosion inhibitor for mild steel in 1 M HCl solution: a complementary experimental, molecular dynamics and quantum mechanics investigation. J. Ind. Eng. Chem. 69, 18 (2019)
K. Rose, B.-S. Kim, K. Rajagopal, S. Arumugam, K. Devarayan, Surface protection of steel in acid medium by Tabernaemontana divaricata extract: physicochemical evidence for adsorption of inhibitor. J. Mol. Liq. 214, 111 (2016)
K.K. Anupama, K. Ramya, A. Joseph, Electrochemical and computational aspects of surface interaction and corrosion inhibition of mild steel in hydrochloric acid by Phyllanthus amarus leaf extract (PAE). J. Mol. Liq. 216, 146 (2016)
P. Mourya, S. Banerjee, M.M. Singh, Corrosion inhibition of mild steel in acidic solution by Tagetes erecta (Marigold flower) extract as a green inhibitor. Corros. Sci. 85, 352 (2014)
P.E. Alvarez, M.V. Fiori-Bimbi, A. Neske, S.A. Brandán, C.A. Gervasi, Rollinia occidentalis extract as green corrosion inhibitor for carbon steel in HCl solution. J. Ind. Eng. Chem. 58, 92 (2018)
M. Rasheed, I. Hussain, S. Rafiq, I. Hayat, A. Qayyum, S. Ishaq, M.S. Awan, Chemical composition and antioxidant activity of quince fruit pulp collected from different locations. Int. J. Food Prop. 21, 2320 (2018)
J.P. Flores-De los Ríos, M. Sánchez-Carrillo, C.G. Nava-Dino, J.G. Chacón-Nava, J.G. González-Rodríguez, E. Huape-Padilla, M.A. Neri-Flores, A. Martínez-Villafañe, Opuntia ficus-indicaExtract as green corrosion inhibitor for carbon steel in 1 M HCl solution. J. Spectrosc. 2015, 1 (2015)
M. Ferdosi Heragh, H. Tavakoli, Electrochemical properties of a new green corrosion inhibitor derived from Prosopis farcta for St37 steel in 1 M hydrochloric acid. Met. Mater. Int. 26, 1654 (2019)
N. Parhizkar, B. Ramezanzadeh, T. Shahrabi, Corrosion protection and adhesion properties of the epoxy coating applied on the steel substrate pre-treated by a sol-gel based silane coating filled with amino and isocyanate silane functionalized graphene oxide nanosheets. Appl. Surf. Sci. 439, 45 (2018)
D. Li, P. Zhang, X. Guo, X. Zhao, Y. Xu, The inhibition of mild steel corrosion in 0.5 M H 2 SO 4 solution by radish leaf extract. RSC Adv. 9, 40997 (2019)
L.C. Go, D. Depan, W.E. Holmes, A. Gallo, K. Knierim, T. Bertrand, R. Hernandez, Kinetic and thermodynamic analyses of the corrosion inhibition of synthetic extracellular polymeric substances. Peer J. Mater. Sci. 2, e4 (2020)
A. Saxena, D. Prasad, R. Haldhar, G. Singh, A. Kumar, Use of Saraca ashoka extract as green corrosion inhibitor for mild steel in 0.5 M H2SO4. J. Mol. Liq. 258, 89 (2018)
N. Asadi, M. Ramezanzadeh, G. Bahlakeh, B. Ramezanzadeh, Utilizing Lemon Balm extract as an effective green corrosion inhibitor for mild steel in 1M HCl solution: a detailed experimental, molecular dynamics, Monte Carlo and quantum mechanics study. J. Taiwan Inst. Chem. Eng. 95, 252 (2019)
M. Mahdavian, A.R. Tehrani-Bagha, K. Holmberg, Comparison of a cationic gemini surfactant and the corresponding monomeric surfactant for corrosion protection of mild steel in hydrochloric acid. J. Surfactants. Deterg. 14, 605 (2011)
F. Askari, E. Ghasemi, B. Ramezanzadeh, M. Mahdavian, Mechanistic approach for evaluation of the corrosion inhibition of potassium zinc phosphate pigment on the steel surface: application of surface analysis and electrochemical techniques. Dyes Pigm. 109, 189 (2014)
L. Guo, B. Tan, W. Li, Q. Li, X. Zheng, I.B. Obot, Banana leaves water extracts as inhibitor for X70 steel corrosion in HCl medium. J. Mol. Liq. 327, 114828 (2021)
A.K. Satapathy, G. Gunasekaran, S.C. Sahoo, K. Amit, P.V. Rodrigues, Corrosion inhibition by Justicia gendarussa plant extract in hydrochloric acid solution. Corros. Sci. 51, 2848 (2009)
E. Baran, A. Cakir, B. Yazici, Inhibitory effect of Gentiana olivieri extracts on the corrosion of mild steel in 0.5 M HCl: electrochemical and phytochemical evaluation. Arab. J. Chem. 12, 4303 (2019)
Y. Qiang, S. Zhang, B. Tan, S. Chen, Evaluation of Ginkgo leaf extract as an eco-friendly corrosion inhibitor of X70 steel in HCl solution. Corros. Sci. 133, 6 (2018)
H. Gerengi, H.I. Sahin, Schinopsis lorentziiextract as a green corrosion inhibitor for low carbon steel in 1 M HCl solution. Ind. Eng. Chem. Res 51, 780 (2011)
D.A. López, S.N. Simison, S.R. de Sánchez, Inhibitors performance in CO2 corrosion. Corros. Sci. 47, 735 (2005)
X. Li, S. Deng, H. Fu, Blue tetrazolium as a novel corrosion inhibitor for cold rolled steel in hydrochloric acid solution. Corros. Sci. 52, 2786 (2010)
H. Tavakoli, T. Shahrabi, M.G. Hosseini, Synergistic effect on corrosion inhibition of copper by sodium dodecylbenzenesulphonate (SDBS) and 2-mercaptobenzoxazole. Mater. Chem. Phys. 109, 281 (2008)
A. Popova, M. Christov, Evaluation of impedance measurements on mild steel corrosion in acid media in the presence of heterocyclic compounds. Corros. Sci. 48, 3208 (2006)
E.A. Noor, Evaluation of inhibitive action of some quaternary N-heterocyclic compounds on the corrosion of Al–Cu alloy in hydrochloric acid. Mater. Chem. Phys. 114, 533 (2009)
S. Garai, S. Garai, P. Jaisankar, J.K. Singh, A. Elango, A comprehensive study on crude methanolic extract of Artemisia pallens (Asteraceae) and its active component as effective corrosion inhibitors of mild steel in acid solution. Corros. Sci. 60, 193 (2012)
M. Cui, Y. Yu, Y. Zheng, Effective Corrosion inhibition of carbon steel in hydrochloric acid by dopamine-produced carbon dots. Polymers. 13, 1923 (2021)
K.F. Khaled, M.M. Al-Qahtani, The inhibitive effect of some tetrazole derivatives towards Al corrosion in acid solution: chemical, electrochemical and theoretical studies. Mater. Chem. Phys. 113, 150 (2009)
E. Alibakhshi, M. Ramezanzadeh, G. Bahlakeh, B. Ramezanzadeh, M. Mahdavian, M. Motamedi, Glycyrrhiza glabra leaves extract as a green corrosion inhibitor for mild steel in 1 M hydrochloric acid solution: experimental, molecular dynamics, Monte Carlo and quantum mechanics study. J. Mol. Liq. 255, 185 (2018)
A. Rahman, M. Ismail, M. Hussain, Inhibition of corrosion of mild steel in hydrochloric acid by Bambusa arundinacea. Int. Rev. Mech. Eng 5, 59 (2011)
M. Husaini, B. Usman, M.B. Ibrahim, Evaluation of corrosion behaviour of aluminum in different environment. Sci Bayero J. Pure Appl. Sci. 11, 88 (2019)
H.S. Gadow, M.M. Motawea, Investigation of the corrosion inhibition of carbon steel in hydrochloric acid solution by using ginger roots extract. RSC Adv. 7, 24576 (2017)
R. Idouhli, A. N’Ait Ousidi, Y. Koumya, A. Abouelfida, A. Benyaich, A. Auhmani, M. Y. Ait Itto, Electrochemical Studies of Monoterpenic Thiosemicarbazones as corrosion inhibitor for steel in 1 M HCl. Int. J. Corros. 2018, 9212705 (2018)
P. Muthukrishnan, B. Jeyaprabha, P. Prakash, Adsorption and corrosion inhibiting behavior of Lannea coromandelica leaf extract on mild steel corrosion. Arab. J. Chem. 10, S2343 (2017)
A. Saxena, D. Prasad, R. Haldhar, G. Singh, A. Kumar, Use of Sida cordifolia extract as green corrosion inhibitor for mild steel in 0.5 M H2SO4. J. Environ. Chem. Eng. 6, 694 (2018)
A. Dehghani, G. Bahlakeh, B. Ramezanzadeh, M. Ramezanzadeh, Potential role of a novel green eco-friendly inhibitor in corrosion inhibition of mild steel in HCl solution: detailed macro/micro-scale experimental and computational explorations. Constr. Build. Mater. 245, 118464 (2020)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Golafshani, M.G., Tavakoli, H. Corrosion and Electrochemical Characterization of St37 in the Presence of Quince Extract as a Green and Sustainable Inhibitor. Electrocatalysis 14, 279–293 (2023). https://doi.org/10.1007/s12678-022-00788-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12678-022-00788-6