Abstract
Quality of groundwater is assessed from a part of Prakasam district, Andhra Pradesh, India. Groundwater samples collected from thirty locations from the study area were analysed for pH, electrical conductivity (EC), total dissolved solids (TDS), calcium (Ca2+), magnesium (Mg2+), sodium (Na+), potassium (K+), bicarbonate (\( {\text{HCO}}_{3}^{ - } \)), chloride (Cl−), sulphate (\( {\text{SO}}_{4}^{2 - } \)), nitrate (\( {\text{NO}}_{3}^{ - } \)) and fluoride (F−). The results of the chemical analysis indicate that the groundwater is alkaline in nature and are mainly characterized by Na+–\( {\text{HCO}}_{3}^{ - } \) and Na+–Cl− facies. Groundwater chemistry reflects the dominance of rock weathering and is subsequently modified by human activities, which are supported by genetic geochemical evolution and hydrogeochemical relations. Further, the chemical parameters (pH, TDS, Ca2+, Mg2+, Na+, \( {\text{HCO}}_{3}^{ - } \), Cl−, \( {\text{SO}}_{4}^{2 - } \), \( {\text{NO}}_{3}^{ - } \) and F−) were compared with the drinking water quality standards. The sodium adsorption ratio, percent sodium, permeability index, residual sodium carbonate, magnesium ratio and Kelly’s ratio were computed and USSL, Wilcox and Doneen’s diagrams were also used for evaluation of groundwater quality for irrigation. For industrial purpose, the pH, TDS, \( {\text{HCO}}_{3}^{ - } \), Cl− and \( {\text{SO}}_{4}^{2 - } \) were used to assess the impact of incrustation and corrosion activities on metal surfaces. As a whole, it is observed that the groundwater quality is not suitable for drinking, irrigation and industrial purposes due to one or more chemical parameters exceeding their standard limits. Therefore, groundwater management measures were suggested to improve the water quality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Drinking water is a basic need for everybody for survival of life. Because of uncertainty in monsoon, usage of groundwater has increased many folds, including drinking, irrigation and industrial purposes. Groundwater is a natural replenishable resource, which is safer than surface water. However, human activities can contaminate aquifers (Todd 1980; Li 2016; Li et al. 2017). And, over withdrawal of groundwater leads to decline of its water levels and consequently it becomes poor in quality due to increase of residence time of water with aquifer material and leakage of poor quality water (Wu et al. 2015). On the other hand, the occurrence of toxic minerals in soils as well as in rocks is also responsible for groundwater contamination (Suresh et al. 2007; Li et al. 2016a; Nagaraju et al. 2016).
In recent time, considerable research has been focused on assessment of groundwater quality for drinking, irrigation and industrial purpose elsewhere (Li et al. 2012, 2013; Wu et al. 2011, 2015). Al-Shaibani (2008) observed the impact of protection measures on groundwater quality in a part of western Saudi Arabia by a hydrochemical study. Senthilkumar et al. (2008) and Srinivasamoorthy et al. (2011) reported that the geogenic and anthropogenic sources play a key role for variation of groundwater quality in a part of Cuddalore, Tamilnadu, India. Manjusree et al. (2009) and Sarath Prasanth et al. (2012) assessed the quality of groundwater for drinking and agricultural purposes in some part of Alappuzha district, Kerala, India. Bhavana and Devi (2011) evaluated the groundwater quality in the region of Guhagar, Maharashtra, India. Subba Rao et al. (2012a) evaluated the geochemistry and quality of groundwater of Gummanampadu Sub-basin, Guntur District, Andhra Pradesh, India. Reddy (2013) explained the hydrogeochemical characteristics in groundwater of the southeastern part of Prakasam district, Andhra Pradesh, India. Pophare et al. (2014) assessed the influence of over-exploitation on the quality of groundwater. Tiwari and Singh (2014), Sajil Kumar et al. (2014) and Venkataramanan et al. (2014) measured the hydrochemistry and groundwater quality in the regions of Pratapgarh (Uttara Pradesh), South Chennai (Tamilnadu) and Muthupet (Tamilnadu), India, respectively. Li et al. (2016a) assessed the hydrochemical characteristics and groundwater quality for drinking and irrigation purposes in and around Hua County, China. Nagaraju et al. (2016) evaluated the hydrogeochemistry of groundwater in Vinukonda region, Guntur district, Andhra Pradesh, India. Ghalib (2017) assessed the factors responsible for groundwater chemistry and evaluated it for drinking and irrigation utilities in East Wasit province, Central Iraq. Nag and Das (2017) explained the groundwater quality from a part of Bankura District, West Bengal, India. Nageswara Rao et al. (2017) assessed the geochemical evolution of groundwater in the Western Delta region of River Godavari, Andhra Pradesh, India. Su et al. (2017) evaluated the groundwater quality and health risks from contamination in the north edge of the loess plateau, Yulin City, Northwest China.
The chemical composition of groundwater is a measure of its suitability as a source of water for human consumption, irrigation and industrial purposes. The variation of quality of groundwater depends upon the water–soil–rock interactions and source of various pollutants. And the excess withdrawal of groundwater can also alter the natural groundwater quality. So, the protection management measures are required for improving the groundwater quality. Since the groundwater is so important for sustainable development of society, the evaluation of groundwater quality in every part of the country is essential for its better management. Therefore, the focus of the present paper is (a) on assessment of controlling factors of groundwater quality and (b) on examination of the groundwater quality for drinking, irrigation and industrial purposes.
Study area
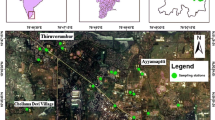
The study area (15°24′–15°–39′30″N and 80°15′–80°35″E) is located in a part of Prakasam District, Andhra Pradesh, India (Fig. 1), which covers an area about 325 km2 with a population of 500,000. The area falls in the Survey of India toposheets 66 A/2 and 66 A/3. It experiences a semi-arid climate. The minimum and maximum temperatures are 28 and 39 °C, respectively. The annual rainfall is about 910 mm. The southwest monsoon (June–September) contributes 69%, the northeast monsoon (October–December) 29% and the remaining winter and summer seasons 12% of the total rainfall. The river Gundlakamma flows through the study area. The drainage is of dendritic to sub-dendritic type and its density is mostly less than 0.4 km/km2 (CGWB 2013).
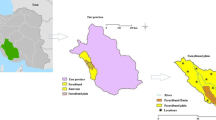
The slope of the study area is in the direction of southeast. The silty clay soils are the dominated type. The CaCO3 concretions (locally known as kankar) occur in the soil cover. The study area is underlain by Precambrian formations (Charnockites of the Eastern Ghats Super-group and unclassified Metamorphic Super-group of the Archaeans with younger intrusive bodies), Upper Gondwana sequence of Lower Cretaceous age and Quaternary Formations (Fig. 2). Pyroxene granulites come under the Charnockite Group (CGWB 2013). Migmatised quartzo-felspathic-gneiss/schist associated with the amphibolites come under the unclassified metamorphic rocks. The rocks are intensely migmatised at places. The rocks contain the quartz, plagioclase feldspar, orthoclase feldspar, hornblende, hypersthene, sillimanite, garnet, apatite and biotite. They show a strike of NE–SW to NW–SE with moderate to steep dip. Sandstones belong to the Upper Gondwana formations. Laterite, brown silty clay, black silty clay, brown silty sand, brown sand and white to grey sand come under the Quaternary deposits.
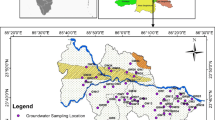
The Precambrian rocks lack primary porosity and hydraulic conductivity. However, they become aquifers by weathering of the rocks and tectonic forces developed in the sub-surface, which form the weathered rock and fractured rock portions. Thickness of the weathered zone is from 3 to 6 m, while the fractured rock is in between 5 and 35 m. Extraction of groundwater is through shallow wells and deep wells. Mostly the former wells extend up to a depth of weathered rock zone, while the depth of latter wells is up to the fractured rock portion. The depth to groundwater level is from 3 to 16 m below ground level (Fig. 3). In general, the recharge area is at upstream side, which is in the direction of northwest and the discharge area is at downstream side, which is in the direction of southwest. The irrigation wells are pumped at a discharge of about 4 L/s to meet the demand of high agricultural production (Reddy et al. 2015). The average yield of wells is 50 and 110 m3/day in the weathered and fractured rock portions, respectively (CGWB 2013). In the river and coastal alluvium, it is 425 and 250 m3/day, respectively. The quality of groundwater is not generally good, which is mostly brackish.
Agriculture is the main occupation of the people in the study area. Irrigation is intensive and long-term practice. Applications of soil amendments like gypsum for improving the soil conditions and chemical fertilizers like potash, phosphate, nitrogen and sulphate varieties for higher crop yields are not uncommon. Important commercial crops are rice, groundnuts, chillies, tobacco, cotton, etc.
Materials and methods
Groundwater samples were collected in half-litre polythene bottles from 30 locations, after the bottles were soaked in 1:1 HCl for 24 h and were rinsed with distilled water followed by deionised water, during summer 2012 (Fig. 1). The bottles were tightly capped without air gap. Immediately, after collection of the water, the groundwater samples were shifted to laboratory for chemical analysis, following the standard water quality procedures of American Public Health Association (APHA 2012).
The groundwater samples collected from the field were analysed for pH, electrical conductivity (EC), total dissolved solids (TDS), total hardness (TH), calcium (Ca2+), magnesium (Mg2+), sodium (Na+), potassium (K+), bicarbonate (\( {\text{HCO}}_{3}^{ - } \)), chloride (Cl−), sulphate (\( {\text{SO}}_{4}^{2 - } \)), nitrate (\( {\text{NO}}_{3}^{ - } \)) and fluoride (F−), following the standard water quality procedures (APHA 2012). The pH and EC were measured in the field, using their portable meters. The EC was used to calculate the concentration of TDS, following the procedure of Hem (1991). The TH (as CaCO3) and Ca2+ were analysed volumetrically, using standard EDTA. The Mg2+ was computed, taking the difference between the TH (as CaCO3) and Ca2+. A flame photometer was used for estimation of Na+ and K+ ions. The \( {\text{HCO}}_{3}^{ - } \) was estimated by titrating with standard HCl. The Cl− was analysed by titrating with standard AgNO3. The \( {\text{SO}}_{4}^{2 - } \) was determined, using turbidimetric procedure, the \( {\text{NO}}_{3}^{ - } \), using colorimetric method and F−, using ion analyzer. The units of all chemical variables (except pH and EC) are expressed in milligrammes per litre (mg/L). The EC is expressed in micro-mhos per centimetre (µS/cm) at 25 °C.
Ionic-balance-error (IBE) between the total cations (Ca2+, Mg2+, Na+ and K+) and the total anions (\( {\text{HCO}}_{3}^{ - } \), Cl−, \( {\text{SO}}_{4}^{2 - } \), \( {\text{NO}}_{3}^{ - } \) and F−) was checked for accuracy of each groundwater sample (Eq. 1). The computed IBE was observed to be within the acceptable limit of ± 5% (Domenico and Schwartz 1990):
Results and discussion
pH, electrical conductivity and total dissolved solids
The pH decides the acidic or basic nature of water quality, which is controlled by CO2–\( {\text{CO}}_{3}^{ - } \)–\( {\text{HCO}}_{3}^{ - } \) equilibrium (Hem 1991). It varies from 7.2 to 8.3 with a mean of 7.81 in the study area (Table 1), indicating a prevailing condition of alkaline environment. The EC is in between 1505 and 4980 with a mean of 2983.50 µS/cm. Since it is a measure of a material’s ability to conduct an electric currents, the difference of it indicates a wide variation of salts present in the groundwater. The degree of salt content is expressed in terms of TDS, which is in the range of 995–3290, being a mean of 1884.27 mg/L in the study area. The TDS is classified as fresh, if it is less than 1000 mg/L; brackish, if it is in between 1000 and 10,000 mg/L; saline, if it varies from 10,000 to 100,000 mg/L; and brine, if it is more than 100,000 mg/L. Accordingly, the quality of groundwater comes under fresh (3.33%) and brackish (96.67%) in the present study area. The difference in the concentration of TDS indicates a wide variation in the geochemical processes.
Major cations
The concentration of Ca2+ is in between 55 and 90 with a mean of 65.67 mg/L, while that of Mg2+ is from 42 to 105 with a mean of 76.87 mg/L (Table 1). The contributions of Ca2+ and Mg2+ to the total cations are 5.48–20.49% (12.48%) and 13.06–31.51% (22.34%), respectively. The higher contribution of Mg2+ than that of Ca2+ is a result of ferromagnesium minerals, ion exchange between Na+ and Ca2+, and precipitation of CaCO3 (Hem 1991). And, drainage wastewaters are also the source of Mg2+ (Todd 1980). The Na+ content is from 185 to 823 with a mean of 444.73 mg/L, contributing 47.21–76.23% (60.59%) to the total cations. The higher contribution of Na+ than that of Ca2+ to the total cations is due to ion exchange and/or precipitation of CaCO3. The occurrence of CaCO3 concretions in the soil cover supports the precipitation of CaCO3, which is formed due to semi-arid climate of the study area. In contrast to the other concentrations of ions among the cations, the K+ content is observed to be low, which is in between 12 and 121 with a mean of 54.33 mg/L. This is because of more resistance of potash feldspars to chemical weathering and fixation of K+ on clay products (Hem 1991). However, its higher concentration could be due to application of chemical fertilizers (potash variety), since there is no other source of it in the study area. The contribution of it to the total cations varies from 1.80 to 7.20% (4.59%).
It is significant to note that the higher contribution of Na+ among the cations is caused by silicate weathering and/or dissolution of soil salts stored by evaporation and anthropogenic origin (Stallard and Edmond 1983; Meyback 1987; Subba Rao 2002; Li et al. 2016b), such as agricultural activities, poor drainage conditions, etc. (Todd 1980).
Major anions
The \( {\text{HCO}}_{3}^{ - } \) content is observed to be 480–1025 with a mean of 731.33 mg/L (Table 1), which contributes 33.77–56.47% (44.09%) to the total anions. The computed average value of pCO2 is 10−1.92 atmosphere (atm), which is significantly higher than that of the atmospheric CO2 (10−3.5 atm). It suggests that the groundwater system is open to soil CO2. This is a result of decay of organic matter and root respiration, which in turn, combines with rainwater (H2O) to form \( {\text{HCO}}_{3}^{ - } \) (Eqs. 2, 3). The higher \( {\text{HCO}}_{3}^{ - } \) in water indicates a dominance of mineral dissolution (Stumm and Morgan 1996):
The concentration of Cl− varies between 215 and 1060 with a mean of 568.83 mg/L (Table 1). It contributes 38.86–63.73% (50.72%) to the total anionic concentration. Generally, it derives mainly from non-lithological source (poor sanitary conditions, irrigation-return-flows, chemical fertilizers, etc.). Further, the contributions of Na+ and Cl− to the total anions are more than that of others due to their higher solubility nature in water (Hem 1991). Thus, the computed saturation index of NaCl (− 2.06) also supports the dissolved state of halite. The content of \( {\text{SO}}_{4}^{2 - } \) is observed to be 22–125 with a mean of 71.47 mg/L. The contribution of \( {\text{SO}}_{4}^{2 - } \) is 3.08–7.56% (5.19%) to the total cations. The applications of soil amendments like gypsum and chemical fertilizers like sulphate variety are expected to be caused for \( {\text{SO}}_{4}^{2 - } \) content in the groundwater, since no lithological source of \( {\text{SO}}_{4}^{2 - } \) ion is observed from the study area.
The value of \( {\text{NO}}_{3}^{ - } \) is from 31 to 96 with a mean of 56.20 mg/L (Table 1), which contributes 1.82–4.81% (3.33%) to the total anions. Like Cl−, the \( {\text{NO}}_{3}^{ - } \) is also a non-lithological source (Hem 1991). In natural conditions, it does not exceed 10 mg/L in water (Cushing et al. 1973). Thus, the higher \( {\text{NO}}_{3}^{ - } \) content beyond this limit is an indication of man-made pollution (Wu and Sun 2016). Poor sanitary conditions and indiscriminate usage of higher fertilizers (nitrogen variety) for crop-yields are responsible for more concentration of \( {\text{NO}}_{3}^{ - } \) in the groundwater.
In the groundwater, the F− content is from 0.70 to 2.80 with a mean of 1.34 mg/L (Table 1). Its contribution ranges from 0.16 to 0.33% (0.25%) to the total anions. Geogenic (apatite, hornblende, biotite and clays) and anthropogenic (chemical fertilizers like phosphate variety) sources are the key factors for F− content under the alkaline condition in the study area. Similar observations have been reported by Subba Rao et al. (2014, 2016).
Hydrogeochemical facies
The distribution and genesis of principal groundwater types along the water flow-paths can be explained by hydrogeochemical facies (Seaber 1962; Back 1966; Subba Rao et al. 2012b). Eight types of the hydrogeochemical facies are observed from the study area (Table 2). They are (a) Na+ > Mg2+ > Ca2+ > K+: \( {\text{HCO}}_{3}^{ - } \) > Cl− > \( {\text{SO}}_{4}^{2 - } \), (b) Na+ > Mg2+ > Ca2+: \( {\text{HCO}}_{3}^{ - } \) > Cl−, (c) Na+ > Mg2+ > Ca2+: \( {\text{HCO}}_{3}^{ - } \) > Cl− > \( {\text{SO}}_{4}^{2 - } \), (d) Na+ > Mg2+ > Ca2+ > K+: \( {\text{HCO}}_{3}^{ - } \) > Cl−, (e) Na+ > Mg2+ > Ca2+: Cl− > \( {\text{HCO}}_{3}^{ - } \) > \( {\text{SO}}_{4}^{2 - } \), (f) Na+ > Mg2+ > Ca2+: Cl− > \( {\text{HCO}}_{3}^{ - } \), (g) Na+ > Mg2+ > Ca2+ > K+: Cl− > \( {\text{HCO}}_{3}^{ - } \) > \( {\text{SO}}_{4}^{2 - } \) and (h) Na+ > Mg2+ > Ca2+ > K+: Cl− > \( {\text{HCO}}_{3}^{ - } \).
Most groundwater samples (23.34%) are represented by Na+ > Mg2+ > Ca2+ > K+: \( {\text{HCO}}_{3}^{ - } \) > Cl− > \( {\text{SO}}_{4}^{2 - } \) facies (Table 2). Twenty per cent of the groundwater samples are shown by Na+ > Mg2+ > Ca2+: Cl− > \( {\text{HCO}}_{3}^{ - } \) facies. Approximately 13% of the groundwater samples belong to two types of facies, which are Na+ > Mg2+ > Ca2+: Cl− > \( {\text{HCO}}_{3}^{ - } \) > \( {\text{SO}}_{4}^{2 - } \) and Na+ > Mg2+ > Ca2+ > K+: Cl− > \( {\text{HCO}}_{3}^{ - } \) > \( {\text{SO}}_{4}^{2 - } \). Similarly, 10% of the groundwater samples are characterized by two types of facies, which are Na+ > Mg2+ > Ca2+: \( {\text{HCO}}_{3}^{ - } \) > Cl− and Na+ > Mg2+ > Ca2+: \( {\text{HCO}}_{3}^{ - } \) > Cl− > \( {\text{SO}}_{4}^{2 - } \). About 7% of the groundwater samples are represented by Na+ > Mg2+ > Ca2+ > K+: Cl− > \( {\text{HCO}}_{3}^{ - } \) facies. The rest (3.33%) are shown by Na+ > Mg2+ > Ca2+ > K+: \( {\text{HCO}}_{3}^{ - } \) > Cl− facies.
It is significant to note that all groundwater samples show commonly the Na+ > Mg2+ > Ca2+ facies among the cations (Table 2). But, 46.67% of the total groundwater samples show the K+ ion, which is in addition to the common cation related facies. Further, it is also noted that the 46.67% of the groundwater samples have the \( {\text{HCO}}_{3}^{ - } \) > Cl− facies, while the 53.33% of the groundwater samples show the Cl− > \( {\text{HCO}}_{3}^{ - } \) facies among the anions. Here also, 60% of the total groundwater samples are shown by \( {\text{SO}}_{4}^{2 - } \) as the additional ion in their anion facies. Therefore, the K+ and \( {\text{SO}}_{4}^{2 - } \) are the additional contributions of ions to the common facies, which are the result of anthropogenic (agro-chemicals) impact on the groundwater system. Otherwise, all groundwater samples should have the same facies. Further, the area represented by \( {\text{HCO}}_{3}^{ - } \) ion can be classified as a recharge zone and the area dominated by Cl− ion as a discharge zone (Ophori and Toth 1989).
Geochemical evolution of groundwater quality
Chebotarev’s genetic geochemical evolution
According to the genetic geochemical evolution of groundwater quality (Chebotarev 1955; Qian and Li 2011), the chemical quality of groundwater is classified into three major groups (Table 3). They are (a) \( {\text{HCO}}_{3}^{ - } \), (b) \( {\text{SO}}_{4}^{2 - } \) and (c) Cl−. The first major group is divided as I, II and III under the genetic water types of \( {\text{HCO}}_{3}^{ - } \), \( {\text{HCO}}_{3}^{ - } \)–Cl− and Cl−–\( {\text{HCO}}_{3}^{ - } \); the second major group as IV and V under the genetic water types of \( {\text{SO}}_{4}^{2 - } \)–Cl− and \( {\text{SO}}_{4}^{2 - } \); and the last major group as III, IV and VI under the genetic water types of Cl−–\( {\text{HCO}}_{3}^{ - } \), Cl−–\( {\text{SO}}_{4}^{2 - } \) and Cl−, respectively, following the recharge zone to discharge zone.
In the present study area, the groundwater samples are observed from the divisions of I and II of the major group of \( {\text{HCO}}_{3}^{ - } \) (Table 3). Genetically, they belong to \( {\text{HCO}}_{3}^{ - } \) and \( {\text{HCO}}_{3}^{ - } \)–Cl− water types in 56.67 and 43.33% of the total groundwater samples. The former genetic type is a result of intensive water flushing caused by good drainage conditions, leading to shorter contact of water with the aquifer materials. Thus, the groundwater shows the lower salt content (TDS: 1460 mg/L). The latter genetic type is caused by influence of inadequate water flushing due to poor drainage conditions. This leads to longer residence of water in the sub-surface materials and thereby its quality of groundwater has the higher salt content (TDS: 2668. 85 mg/L).
Langelier and Ludwig’s geochemical evolution
The evolution of groundwater quality is also assessed to distinguish fresh water from saline water, using Langelier and Ludwig’s graphical diagram (Langelier and Ludwig 1942). The groundwater sample 7 falls in the Group I, relating to the Ca2+–Mg2+–\( {\text{HCO}}_{3}^{ - } \) type (Fig. 4), which indicates a fresh water quality (TDS: 995 mg/L). This is a result of water–rock interaction. The rest of the groundwater samples (1–6 and 8–30) are observed from Group II, where their quality is dominated by Na+ + K+ and Cl−+ \( {\text{SO}}_{4}^{2 - } \) over the Ca2+ + Mg2+ and \( {\text{HCO}}_{3}^{ - } \) + \( {\text{CO}}_{3}^{2 - } \) ions and their TDS varies from 1095 to 3290 mg/L (2017 mg/L) due to influence of anthropogenic activities on the groundwater body. This water quality comes under brackish type (TDS: 1000–10,000 mg/L). Thus, the changes in evaluation of groundwater quality from Group I and Group II conclude that the chemistry of groundwater is mainly controlled by geogenic origin (former Group), which is subsequently modified by anthropogenic origin (latter Group).

(after Langelier and Ludwig 1942)
Geochemical evolution
Hydrogeochemical relations
To assess the origin of dissolved salts and the geochemical processes involved in the groundwater system, hydrogeochemical relations are widely used (Sarin et al. 1989; Subba Rao 2017). The sampling points of groundwater of the study area are plotted in Ca2+ + Mg2+ versus \( {\text{HCO}}_{3}^{ - } \) diagram (Fig. 5a). Most groundwater sampling points are observed below the equiline of Ca2+ + Mg2+: \( {\text{HCO}}_{3}^{ - } \). This is caused by excess of alkalinity (\( {\text{HCO}}_{3}^{ - } \)), which has been balanced by alkalies (Na+ and K+). Further, the chemical data of groundwater samples fall below the uniline of Ca2+ + Mg2+ versus total cations (Fig. 5b). This indicates an increasing contribution of alkalies to the total cations caused by rock weathering.
All groundwater sampling points in a plot of Na+ + K+ versus total cations are observed below the equiline and above the 0.50 of Na+ + K+: total cations (Fig. 5c). This is a result of supply of cations through the rock weathering and/or soil salts stored by evaporation (Stallard and Edmond 1983; Meyback 1987; Subba Rao 2002). Further, there is some deviation in the spreading trend in a plot of Na+ + K+ versus Cl− + \( {\text{SO}}_{4}^{2 - } \)(Fig. 5d). This is expected due to different origin of these ions sources. For instance, the observed groundwater sampling points falling just below the uniline of Na+ + K+: Cl− + \( {\text{SO}}_{4}^{2 - } \) may be responsible for the source of geogenic origin, while others, which are far below the equiline of Na+ + K+: Cl− + \( {\text{SO}}_{4}^{2 - } \), are due to anthropogenic source.
The chemical data fall below the theoretical line of Na+ versus Cl− in most groundwater samples (Fig. 6a). Because of release of Na+ from the rock weathering (Meyback 1987; Stumm and Morgan 1996), the groundwater would have \( {\text{HCO}}_{3}^{ - } \) as a dominant ion (Rogers 1989). Further, most groundwater sampling points are observed above the equiline of Na+: \( {\text{HCO}}_{3}^{ - } \) (Fig. 6b). Thus, the groundwater is characterized by Na+–\( {\text{HCO}}_{3}^{ - } \) facies. Whereas, the groundwater sampling points, which fall below the theoretical line of Na+: \( {\text{HCO}}_{3}^{ - } \), suggest that the Na+ is also contributed from the anthropogenic source, which support the above views.
Since the concentration of Na+ is higher than that of Ca2+ (Fig. 6c), it indicates an ion exchange (Li et al. 2014a, b, c). This is further verified by plotting of the chemical data of groundwater sampling in a diagram Na+: Cl− versus EC (Fig. 6d). All points are spread horizontally, but they show some deviation in their trend. It suggests that the change in the ratio of Na+: Cl− with an increase of EC is not only due to influence of ion exchange, but also due to impact of evaporation by the occurrence of clay in the weathered rock zone and the prevailing condition of semi-arid climate in the study area.
Point source and non-point source pollution
Pollution is the introduction of contaminants into the environment (air, water or soil). The contaminants are classified into two types. They are the sources of natural (geogenic) and artificial (anthropogenic) origins. If they come from a single location, it is called point source pollution. If they come over a large or widespread area, it is considered non-point source pollution. In view of this, the sources of geogenic and anthropogenic origins influencing the groundwater quality discussed in the study area come under point source pollution.
Suitability of groundwater quality
Drinking purpose
A quality criterion is generally based on a water intake per person per day. Drinking water should be uncontaminated. If there is any contamination in water, it should be treated before its consumption. To assess the water quality for drinking purpose, WHO (2011) and BIS (2012) have laid down a certain criteria. Accordingly, the top priority should be given to the highest desirable (or acceptable) limits prescribed for drinking purpose, while the maximum permissible (allowable) limits may be extended, if there is no any alternative water availability (Subba Rao 2017). The results of the groundwater quality of the present study are compared and discussed with the highest desirable limits (Table 4).
If the pH is not within the highest desirable limit of 6.5–8.5 (Table 4), it damages mucous membrane (eyes, nose, mouth, abdomen, anus, etc.). The pH (7–8.2) in all groundwater samples are observed to be within its safe limit. Since the TDS (995–3290 mg/L) is 1.99–6.58 times higher than its desirable limit of 500 mg/L in all groundwater samples, it decreases palatability and causes gastrointestinal irritation in the consumers (WHO 2011; BIS 2012).
The Ca2+ is an important ion to develop proper bone growth. The observed Ca2+ content (55–90 mg/L) is higher than its standard limit of 75 mg/L in 3.33% of the total groundwater sample (Table 4). The concentration of Mg2+ (52–105 mg/L) is 1.73–3.5 times higher than its prescribed limit of 30 mg/L in all total groundwater samples. The concentration of Na+ (185–823 mg/l) is 1.03–4.12 times higher than its recommended limit of 200 mg/L in 90% of the total groundwater samples. It causes hypertension (Holden 1970).
Bicarbonate is essential in human body for food digestion. The concentration of \( {\text{HCO}}_{3}^{ - } \) (480–1025 mg/L) is 1.6–3.42 times higher than its desirable limit of 300 mg/L in all groundwater samples (Table 4). However, the \( {\text{HCO}}_{3}^{ - } \) has no known adverse health effects on human health. Generally, the Cl− and \( {\text{SO}}_{4}^{2 - } \) ions deteriorate the quality of drinking water at larger extent (WHO 2011; BIS 2012). Thus, the threshold limits of Cl− and \( {\text{SO}}_{4}^{2 - } \) for drinking water are 250 and 200 mg/L, respectively. The concentration of Cl− (215–1060 mg/L) is observed to be 1.02–4.24 times higher than its safe in 90% of the total groundwater samples, while the concentration of \( {\text{SO}}_{4}^{2 - } \) (22–125 mg/L) is below its prescribed limit in all groundwater samples. The concentration of \( {\text{NO}}_{3}^{ - } \)(31–96 mg/L) is 1.02–2.13 times higher than its desirable limit of 45 mg/L in 73.33% of the total groundwater samples. It causes a methemoglobinemia, which is a dangerous fatal disease, especially in infants of less than 4 months old (Bouwer 1978). The concentration of F− is essential for proper development of bones and teeth. In the present study area, the F− (0.7–2.8 mg/L) is 1.07–1.87 times higher than its highest desirable limit of 1.50 mg/L in 43.33% of the total groundwater samples. It causes fluorosis (WHO 2011; BIS 2012).
Irrigation purpose
Higher concentrations of ions dissolved in irrigation water affect soil structure and consequently plant growth by reducing osmotic activity in plant cells (Saleh et al. 1999). It prevents water from reaching the branches and leaves, thus reducing the agricultural output. Salinity hazard, sodium hazard, per cent sodium, permeability index, residual sodium carbonate, magnesium ratio and Kelly’s ratio are widely used for assessment of water quality for irrigation.
Poor drainage conditions cause salinity hazard (C), which forms saline soils, causing a crop loss. Sodium hazard (S) makes soil compact and impervious due to exchange of Na+ for Ca+2 in soils (Todd 1980). Thus, it reduces plant growth. The former hazard is expressed in terms of EC and the latter hazard in terms of sodium adsorption ratio (SAR) and percent sodium (%Na+), where their ions are expressed in milliequivalent per litre (meq/L; Eqs. 4, 5):
Salinity hazard and sodium hazard
The United States Soil Laboratory Staff (USSLS)’s diagram (Richards 1954) classifies the water quality into 16 zones to assess the degree of suitability of water for irrigation (Fig. 7), in which the salinity hazard (C) is divided into four sub-zones, such as low salinity hazard (C1: < 250 μS/cm), medium-salinity hazard (C2: 250–750 μS/cm), high-salinity hazard (C3: 750–2250 μS/cm) and very-high-salinity hazard (C4: > 2250 μS/cm), considering them as good, moderate, poor and very poor water classes, respectively. Similarly, the sodium hazard (S) is classified into four sub-zones. They are low-sodium hazard (S1: < 10), medium-sodium hazard (S2: 10–18), high-sodium hazard (S3: 18–26) and very-high sodium hazard (S4: > 26), considering them as good, moderate, poor and very poor classes, respectively (Table 6).

(after Richards 1954)
Groundwater quality for irrigation
The EC varies from 1505 to 4980 with a mean of 2.983.50 μS/cm (Table 1). The SAR is from 3.89 to 14.97 with a mean of 8.64, respectively (Table 5). The integrated impact of EC and SAR on plant growth is shown in USSL’s diagram (Fig. 7). About 43% (43.33%) of the groundwater samples are observed from the C3S1 zone, 10% of the samples from the C4S2 and C4S3 zones, and 36.67% of the samples from the C4S4 zone (Table 6). This indicates a gradual increase of salinity hazard and sodium hazard for irrigation. Thus, the crops of semi-tolerant to salt tolerant can be selected, following the special soil treatment methods.
Per cent sodium
If %Na+ increases, it reduces soil permeability and consequently plant growth (Li et al. 2014a). Thus, the soils require special treatment like gypsum to increase their permeability. In general, %Na+ should not exceed more than 60 in water used for irrigation. The %Na+ can be computed, following Eq. (5). The groundwater shows %Na+ from 52.04 to 79.39 with a mean of 65.18 in the study area (Table 5). Therefore, the quality of groundwater in 36.67% of the total samples is suitable and in 63.33% is not suitable (Table 7).
Electrical conductivity and %Na+
Wilcox (1955) proposed a diagram with respect to a combination of EC and %Na+ for judging suitability of water quality for irrigation (Fig. 8). The diagram is divided into five zones, which are excellent to good, good to permissible, permissible to doubtful, doubtful to unsuitable and unsuitable, with increasing salinity hazard and sodium hazard for irrigation. Broadly, they are considered as excellent, good, moderate, poor and very poor water qualities, respectively, for irrigation (Table 8).

(after Wilcox 1955)
Groundwater quality for irrigation
Ten per cent of the groundwater samples fall in good to permissible zone, 26.67% of the groundwater samples in permissible to doubtful zone, 16.67% of the groundwater samples in doubtful to unsuitable zone and 46.66% of the groundwater samples in undoubtful zone for irrigation (Table 8).
Generally, the agricultural out-put is low in the area, where the water quality is doubtful to unsuitable for irrigation purpose due to excess sodium salts in it. This is a result of osmotic effects on soil–plant system (Saleh et al. 1999). When the Na+ is more than Ca2+ in soils, it reduces permeability and consequently plant growth. Thus, special soil treatment is necessary.
Permeability index
Soil permeability plays a vital role in plant growth. If it is low, it does not support to plant growth. It is greatly influenced by Na+, Ca2+, Mg2+ and \( {\text{HCO}}_{3}^{ - } \) contents of soil and hence is affected by long-term use of irrigation water, with high salt content (Li et al. 2014a, 2016a, c). Doneen (1964) expressed the degree of soil permeability in terms of permeability index (PI), which is computed, following Eq. (6), where all ions are expressed in meq/L:
The quality of water can be classified on the basis of PI into three classes (Table 9). They are (a) class I, (b) class II, and (c) class III, which have 100, 75 and 25% maximum permeabilities, respectively. The first class is suitable, the second class II is marginally suitable and the third class is unsuitable for irrigation. The PI varies from 65.63 to 88.72 with a mean of 76.75 in the study area (Table 5). According to the classification of PI, 60% of the groundwater samples come under suitable category (class I) and the rest 40% come under marginally suitable category (class II) for irrigation due to decrease of soil permeability.
With relation of PI to the total concentration (Fig. 9), the groundwater samples 1–11 and 13–20 (63.33%) fall in the Class I (100% maximum permeability) and the remaining samples 12 and 21–30 (36.67%) in the Class II (75% permeability) for irrigation in the study area.

(after Doneen 1964)
Groundwater quality for irrigation
Residual sodium carbonate
Carbonates (\( {\text{HCO}}_{3}^{ - } \) + \( {\text{CO}}_{3}^{2 - } \)) effect on water quality through precipitation of alkaline earths (Ca2+ + Mg2+), which increase Na+ content (Eaton 1950). If they (\( {\text{HCO}}_{3}^{ - } \) + \( {\text{CO}}_{3}^{2 - } \)) are in excess of alkaline earths, the excess carbonates combining with Na+ to form NaHCO3 (Eq. 7). It affects soil structure. This is called residual sodium carbonate (RSC; Eq. 8). The higher the RSC, the greater is the adsorption of Na+ in soil, which reduces soil permeability and consequently plant growth:
The RSC can be classified as suitable, if the RSC is less than 1.25 meq/L; marginally suitable, if the RSC is between 1.25 and 2.50 meq/L; and unsuitable, if the RSC is higher than 2.50 meq/L.
The RSC is from − 0.15 to 6.58 with a mean of 2.58 (Table 5). According to the classification of RSC, about 37% of the groundwater samples come under suitable type, 13% under marginal suitable type and 50% under unsuitable type for irrigation (Table 10) due to gradual increase of Na+ content in soils, which causes low soil permeability.
Magnesium ratio
Generally, Ca2+ and Mg2+ ions maintain a state of equilibrium in water. But, they do not behave equally in soil system. Magnesium damages soil structure, when water contains more Na+ and higher salinity. Generally, a high level of Mg2+ is caused by exchangeable Na+ in irrigated soils. In equilibrium, more Mg2+ affects soil quality by rendering it alkaline. Thus, it affects crop yields. This effect on crop yields is expressed in terms of magnesium ratio (MR; Szaboles and Darab 1964), which is computed as shown in Eq. (9), where all ions are expressed in meq/L:
If MR is more than 50 in water, such water quality is considered to be harmful for irrigation due to its adverse affect on crop yields. Thus, such water quality is unsuitable for irrigation. The MR is in between 53.56 and 77.58 with a mean of 65.10 in the study area (Table 5). The groundwater quality in all samples is unsuitable for irrigation (Table 11), as magnesium damages soil structure, which affects crop yields.
Kelly’s ratio
Kelly’s ratio (KR) is used to classify the irrigation water quality, which is the level of Na+ to measure the levels of Ca2+ and Mg2+ ions (Kelly 1963). The formula for calculating the KR is as follows Eq. (10), where the concentrations of ions are in meq/L.
Based on Kelly’s ratio, the water can be classified as suitable, if KR is less than 1 and unsuitable, if it is more than 1. In the study area, the KR varies from 0.94 to 3.70 with a mean of 1.94 (Table 5). According to the classification of KR, about 3% of the groundwater samples are suitable for irrigation and the rest (96.67%) are not suitable for irrigation (Table 12) due to increase of Na+ against Ca2+ and Mg2+ ions, which decreases soil permeability.
Industrial purpose
Utilization of water for industry is quite diverse within the same industry so that every industry has its own water quality standards. However, many industrial sectors frequently suffer from incrustation and corrosion activities due to poor water quality. The incrustation is a result of deposition of CaCO3 on metal surfaces, while the corrosion is a chemical action on metals that results in metals being eaten away. Therefore, the following generalized water quality criterion has been adopted (Anon 1986) here for deciding the water quality for industrial purpose (Table 13).
-
If water contains \( {\text{HCO}}_{3}^{ - } \) more than 400 mg/L or \( {\text{SO}}_{4}^{2 - } \) more than 100 mg/L, it causes incrustation, and
-
If water contains pH less than 7 or TDS more than 1000 mg/L or Cl− more than 500 mg/L, it causes corrosion.
As per the water quality criteria for industrial purpose, the groundwater quality develops incrustation in 100% of the samples due to high \( {\text{HCO}}_{3}^{ - } \) and in 23.33% of the samples due to high \( {\text{SO}}_{4}^{2 - } \) (Table 13), whereas the quality in 96.67% of groundwater samples can develop corrosion due to high TDS, and in 46.67% of the samples due to high Cl−.
Management measures of groundwater quality
Groundwater is so important for sustainable development of society. The groundwater quality management measures are, thus, mandatory for efficient utilization of water by man. It implies the optimum level of utilization for present and future generations. Once the quality of water is suitable for drinking, it can also be utilized for other purposes, including irrigation and industrial uses. In view of this, the following suggestions were made:
-
The interference of human activities (indiscrimination of disposal of drainage wastes and uncontrolled usage of agricultural chemicals) should be controlled in a more effective and stricter way, which is the most important measure of preventing groundwater contamination.
-
Rainwater harvesting methods can play a major role not only to increase the groundwater levels, but also to improve the groundwater quality. Since the study area has sufficient amount of rainfall and is characterized by enough secondary porosity in the geological formations, it can be possible to arrest the higher amount of rainwater for percolating it through fractures for rising of groundwater levels. Check dams, recharge pits, etc. are the successful rainwater harvesting measures not only to increase the groundwater quantity, but also to reduce the salinity (TDS) and other ions (Ca2+, Na+, \( {\text{HCO}}_{3}^{ - } \), Cl−, \( {\text{NO}}_{3}^{ - } \) and F−) by dilution for their suitability of drinking, irrigation and industrial purposes.
-
Water softening and defluoridation techniques can improve the water quality by reducing the Na+ and F− contents. The former approach is the removal of calcium, magnesium and certain other metal cations in hard water, which is usually achieved using lime softening or ion-exchange resins. The second procedure is the removal of excess fluoride from water, using applications of Nalgonda technique, activated alumina, red mud, montmorillonite and magnesia.
-
Alternative supply of safe drinking water in combination with nutritional diet should be provided to all, until the restoration of public health.
-
Improvement of sub-surface drainage conditions, deep cultivation procedures, reclamation, leaching and drip, or sprinkler irrigation is the important land developmental activities, which improve the soil texture as well as soil salinity and water salinity, for facilitating the irrigation easier (Ayers and Westcot 1985).
-
Establishment of regular groundwater quality monitoring network is essential to know the causes and sources of groundwater contamination from time to time for taking suitable remedial measures, accordingly, in time.
-
Creating public awareness on groundwater quality is a key factor for its effective management.
-
The civic authorities must take responsibility for implementing appropriate management measures to improve the groundwater quality.
Conclusions
The following conclusions were drawn from the study carried out in a part of Prakasam district, Andhra Pradesh, India:
-
Groundwater quality is alkaline in nature. In most groundwater samples, alkalies exceed alkaline earths. The groundwater is characterized by Na+: \( {\text{HCO}}_{3}^{ - } \) and Na+: Cl− facies.
-
Rock weathering is the natural geogenic source influencing the groundwater chemistry. Anthropogenic sources are the secondary origin to alter the natural groundwater quality. This is further supported by hydrogeochemical facies, genetic geochemical evolution and hydrogeochemical relations.
-
Assessment of groundwater quality with respect to the pH, TDS, Ca2+, Mg2+, Na+, \( {\text{HCO}}_{3}^{ - } \), Cl−, \( {\text{SO}}_{4}^{2 - } \), \( {\text{NO}}_{3}^{ - } \) and F− suggests that the water is not suitable for drinking due to one or more chemical parameters, exceeding their highest desirable limits.
-
Suitability of groundwater quality for irrigation is evaluated, considering the sodium adsorption ratio, per cent sodium, permeability index, residual sodium carbonate, magnesium ratio and Kelly’s ratio, and also using USSL, Wilcox and Doneen’s diagrams. According to them, most groundwater samples are unfit for irrigation.
-
For industrial purpose, the pH, TDS, \( {\text{HCO}}_{3}^{ - } \), Cl− and \( {\text{SO}}_{4}^{2 - } \) are used and assessed their impact with respect to incrustation and corrosion activities on metal surfaces. It is observed that the groundwater quality is not even fit for industrial uses.
-
Suitable management measures such as controlling human activities, implementation of rainwater harvesting methods, water and soil treatments, creating public awareness and groundwater quality monitoring network are suggested for improving the groundwater quality.
-
The study suggestions will also help for other regions facing similar situations.
References
Al-Shaibani AM (2008) Hydrogeology and hydrochemistry of a shallow alluvial aquifer, western Saudi Arabia. Hydrogeol J 16:155–165
Anon F (1986) Groundwater and wells, 2nd edn. Johnson Screens, St. Paul
APHA (2012) Standard methods for the examination of water and wastewater, 22nd edn. American Public Health Association, Washington, DC
Ayers RS, Westcot DW (1985) Water quality for agriculture, irrigation and drainage. Paper No. 29. Food and Agriculture Organization of the United Nations, Rome, pp 1–117
Back W (1966) Hydrochemical facies and groundwater flow pattern in northern part of Atlantic Coastal Plain. U.S. Geological Survey Professional Paper 498A
Bhavana NU, Devi NM (2011) Groundwater quality assessment for Guhagar coastal area, Maharashtra, India. J Environ Res Dev 5:880–891
BIS (2012) Drinking water specifications. Bureau of Indian Standards, IS 10500
Bouwer H (1978) Groundwater hydrology. McGraw-Hill Book Company, New York
CGWB (2013) Groundwater brochure, Prakasam district, Central Ground Water Board, Government of India, New Delhi
Chebotarev II (1955) Metamorphism of natural waters in the crust of weathering. Geochim Cosmochim Acta 8:22–48, 137–170, 198–212
Cushing EM, Kantrowitz IH, Taylor KR (1973) Water resources of the Delmarva Peninsular. U.S. Geological Survey Professional Paper 822, Washington DC
Domenico PA, Schwartz FW (1990) Physical and chemical hydrogeology. Wiley, New York
Doneen LD (1964) Notes on water quality in agriculture. Water Science and Engineering Paper 4001, California, Department of Water Sciences and Engineering, University of California
Eaton EM (1950) Significance in carbonate in irrigation water. Soil Sci 69:123–133
Ghalib HB (2017) Groundwater chemistry evaluation for drinking and irrigation utilities in east Wasit province, Central Iraq. Appl Water Sci. https://doi.org/10.1007/s13201-017-0575-8
Hem JD (1991) Study and interpretation of the chemical characteristics of natural water: USGS Professional Paper Book 2254. Scientific Publishers, Jodhpur
Holden WS (1970) Water treatment and examination. J & Churchill Publishers, London
Kelly WP (1963) Use of saline irrigation water. Soil Sci 95:355–439
Langelier WF, Ludwig HF (1942) Graphic method for indicating the mineral character of natural water. J Am Water Works Assoc 34:335–352
Li P (2016) Groundwater quality in western China: challenges and paths forward for groundwater quality research in Western China. Expo Health 8(3):305–310
Li P, Wu J, Qian H (2012) Groundwater quality assessment based on rough sets attribute reduction and TOPSIS method in a Semi-arid Area, China. Environ Mon Assess 184(8):4841–4854
Li P, Wu J, Qian H (2013) Assessment of groundwater quality for irrigation purposes and identification of hydrogeochemical evolution mechanisms in Pengyang County, China. Environ Earth Sci 69(7):2211–2225
Li P, Qian H, Wu J, Chen J, Zhang Y, Zhang H (2014a) Occurrence and hydrogeochemistry of fluoride in shallow alluvial aquifer of Weihe River, China. Environ Earth Sci 71(7):3133–3145
Li P, Wu J, Qian H (2014b) Hydrogeochemistry and quality assessment of shallow groundwater in the Southern Part of the Yellow River Alluvial Plain (Zhongwei Section), China. Earth Sci Res J 18(1):27–38
Li P, Wu J, Qian H, Lyu X, Liu H (2014c) Origin and assessment of groundwater pollution and associated health risk: a case study in an industrial park, northwest China. Environ Geochem Health 36(4):693–712
Li P, Wu J, Qian H (2016a) Hydrochemical appraisal of groundwater quality for drinking and irrigation purposes and the major influencing factors: a case study in and around Hua County, China. Arab J Geosci 9:15
Li P, Wu J, Qian H, Zhang Y, Yang N, Jing L, Yu P (2016b) Hydrogeochemical characterization of groundwater in and around a wastewater irrigated forest in the southeastern edge of the Tengger Desert, Northwest China. Expo Health 8(3):331–348
Li P, Zhang Y, Yang N, Jing L, Yu P (2016c) Major ion chemistry and quality assessment of groundwater in and around a mountainous tourist town of China. Expo Health 8(2):239–252
Li P, Tian R, Xue C, Wu J (2017) Progress, opportunities and key fields for groundwater quality research under the impacts of human activities in China with a special focus on western China. Environ Sci Pollut Res 24(15):13224–13234
Manjusree TM, Sabu Joseph, Jobin Thomas (2009) Hydrogeochemistry and groundwater quality in the coastal sandy clay aquifers of Alappuzha district, Kerala. J Geol Soc India 74:459–468
Meyback M (1987) Global chemical weathering of surficial rocks estimated from river dissolved loads. Am J Sci 287:401–428
Nag SK, Das SH (2017) Assessment of groundwater quality from Bankura I and II Blocks, Bankura District, West Bengal, India. Appl Water Sci. https://doi.org/10.1007/s13201-017-0530-8
Nagaraju A, Balaji E, Thejaswi A (2016) Hydrogeochemical evaluation of groundwater in certain parts of Vinukonda area, Guntur District, Andhra Pradesh, South India. Fresenius Environ Bull 25:1519–1532
Nageswara Rao PV, Appa Rao S, Subba Rao N (2017) Geochemical evolution of groundwater in the Western Delta region of River Godavari, Andhra Pradesh, India. Appl Water Sci 7:813–822
Ophori DU, Toth J (1989) Patterns of groundwater chemistry, Ross Creek Basin, Canada. Groundwater 27:20–26
Pophare AM, Lamsoge BR, Katpatal YB, Nawale VP (2014) Impact of over-exploitation on groundwater quality: a case study from WR-2 Watershed, India. J Earth Syst Sci 123(7):1541–1566
Qian H, Li P (2011) Hydrochemical characteristics of groundwater in Yinchuan plain and their control factors. Asian J Chem 23(7):2927–2938
Reddy AGS (2013) Evaluation of hydrogeochemical characteristics of phreatic alluvial aquifers in southeastern coastal belt of Prakasam district, South India. Environ Earth Sci 68:471–485
Reddy DV, Nagabhushanam P, Madhav T, Chandrakala P, Reddy AGS (2015) Characterization of groundwater contaminant sources in the coastal sand dune aquifer, Prakasam district, A.P., India. Environ Earth Sci. https://doi.org/10.1007/s12665-015-438-z
Richards LA (1954) Diagnosis and improvement of saline and alkalis soils. US Department of Agriculture Handbook 60
Rogers RJ (1989) Geochemical comparison of groundwater in areas of New England, New York and Pennsylvania. Groundwater 27:690–712
Sajil Kumar PJ, Elango L, James EJ (2014) Assessment of hydrochemistry and groundwater quality in the coastal area of South Chennai, India. Arab J Geosci 7:2641–2653
Saleh A, Al-Ruwaih F, Shehata M (1999) Hydrogeochemical processes operating within the main aquifers of Kuwait. J Arid Environ 42:195–209
Sarath Prasanth SV, Magesh NS, Jitheshlal KV, Chandrasekar N, Gangadhar K (2012) Evaluation of groundwater quality and its suitability for drinking and agricultural use in the coastal stretch of Alappuzha District, Kerala, India. Appl Water Sci 2:165–175
Sarin MM, Krishnaswamy S, Dillikumar SBLK, Moore WS (1989) Major-ion chemistry of the Ganga–Brahmaputra river system: weathering processes and fluxes to the Bay of Bengal. Geochim Cosmochim Acta 53:997–1009
Seaber PR (1962) Cation hydrochemical facies of groundwater in the Englishtown Formation, New Jersey. U.S. Geological Survey Professional Paper 450 B, pp 124–126
Senthilkumar G, Ramanathan AL, Nainwal HC, Chidambaram S (2008) Evaluation of the hydrogeochemistry of groundwater using factor analysis in the Cuddalore coastal region, Tamilnadu, India. Indian J Mar Sci 37:181–185
Srinivasamoorthy M, Vasanthavigar S, Chidambaram S, Anandan P, Sharma VS (2011) Characterization of groundwater chemistry in an eastern coastal area of Cuddalore district, Tamilnadu. J Geol Soc India 78:549–558
Stallard RE, Edmond JM (1983) Geochemistry of Amazon River: the influence of the geology and weathering environment on the dissolved load. J Geophys Res 88:9671–9688
Stumm W, Morgan JJ (1996) Aquatic chemistry. Wiley-Interscience, New York
Su H, Kang W, Xu Y, Wang J (2017) Evaluation of groundwater quality and health risks from contamination in the north edge of the loess plateau, Yulin City, Northwest China. Environ Earth Sci. https://doi.org/10.1007/s12665-017-6781-8
Subba Rao N (2002) Geochemistry of groundwater in parts of Guntur District, Andhra Pradesh, India. Environ Geol 41:552–562
Subba Rao N (2017) Hydrogeology: problems with solutions. Prentice Hall India, New Delhi
Subba Rao N, Subrahmanyam A, Ravi Kumar S, Srinivasulu N, Babu Rao G, Surya Rao P, Venktram Reddy G (2012a) Geochemistry and quality of groundwater of Gummanampadu Sub-basin, Guntur District, Andhra Pradesh, India. Environ Earth Sci 67:1451–1471
Subba Rao N, Surya Rao P, Venktram Reddy G, Nagamani M, Vidyasagar G, Satyanarayana NLVV (2012b) Chemical characteristics of groundwater and assessment of groundwater quality in Varaha River Basin, Visakhapatnam District, Andhra Pradesh, India. Environ Mon Assess 184:5189–5214
Subba Rao N, Vidyasagar G, Surya Rao P, Bhanumurthy P (2014) Assessment of hydrogeochemical processes in a coastal region: application of multivariate statistical model. J Geol Soc India 84L:494–500
Subba Rao N, Dinakar A, Surya Rao P, Rao PN, Pandith Madhnure Prasad KM, Sudarshan G (2016) Geochemical processes controlling fluoride-bearing groundwater in the granitic aquifer of a semi-arid region. J Geol Soc India 88:350–356
Suresh S, Dinakar N, Prasad TNVKV, Nagajyothi PC, Damodharam T, Nagaraju A (2007) Effects of a barite mine on groundwater quality in Andhra Pradesh, India. Mine Water Environ 26:119–123
Szaboles I, Darab C (1964) The influence of irrigation water of high sodium carbonate content of soils. In: Proceedings of 8th international congress of ISSS, trans, II, pp 803–812
Tiwari AK, Singh AK (2014) Hydrogeochemical investigation and groundwater quality assessment of Pratapgarh District, Uttar Pradesh. J Geol Soc India 83:329–343
Todd DK (1980) Groundwater hydrology. Wiley, New York
Venkataramanan S, Ramkumar T, Vasudevan S, Ramesh G, Anitha Mary G, Viajayakumar V (2014) Assessment of hydrochemistry of groundwater in Muthupet coastal region, Tamilnadu, India. Int J Appl Environ Sci 4:165–172
WHO (2011) Guidelines for drinking water quality. World Health Organization, Geneva
Wilcox LV (1955) Classification and use of irrigation water. U.S. Department of Agriculture Circular 969, Washington, DC
Wu J, Sun Z (2016) Evaluation of shallow groundwater contamination and associated human health risk in an alluvial plain impacted by agricultural and industrial activities, mid-west China. Expo Health 8(3):311–329
Wu J, Li P, Qian H (2011) Groundwater quality in Jingyuan County, a semi-humid area in Northwest China. Eur J Chem 8(2):787–793
Wu J, Li P, Qian H (2015) Hydrochemical characterization of drinking groundwater with special reference to fluoride in an arid area of China and the control of aquifer leakage on its concentrations. Environ Earth Sci 73(12):8575–8588
Acknowledgements
The author is thankful to the University Grants Commission, Government of India, New Delhi, for financial support to carry out this work under major research project [F.No.39-132/2010 (SR)]. The anonymous reviewers and the editors are also greatly acknowledged for their valuable suggestions and useful comments to improve the quality of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Subba Rao, N. Groundwater quality from a part of Prakasam District, Andhra Pradesh, India. Appl Water Sci 8, 30 (2018). https://doi.org/10.1007/s13201-018-0665-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-018-0665-2