Abstract
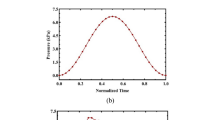
The aorta with its compliance plays a major role in hemodynamics as it saves a portion of ejected blood during systole which is then released in diastole. The aortic compliance decreases with increasing age, which is related to several cardiovascular imparities and diseases. Changes in flow patterns and pressure curves, due to varying aortic compliance, are difficult to investigate in vivo. As a result, the aim of the present work was to develop an in vitro setup enabling standardized investigations on the effect of compliance changes on flow patterns and pressure curves. Therefore an experimental setup with an anatomically correct silicone phantom of the aortic arch was developed, suitable for optical flow measurements under pulsatile inflow conditions. The setup was developed for precise adjustments of different compliances and optical flow measurements. Particle image velocimetry measurements were carried out downstream of the aortic valve in the center plane perpendicular to the valve with compliance adjusted between 0.62 × 10−3 to 1.82 × 10−3 mmHg−1. Preliminary results of the in vitro investigations showed that decreases in compliance results in significant increases in pressure changes with respect to time (dp/dt) and altered pressure curves in the aortic arch. In terms of flow, an increased aortic stiffness lead to higher mean velocities and decreased vortex development in the aortic sinuses. As in vivo validation and translation remains difficult, the results have to be considered as preliminary in vitro insights into the mechanisms of (age-related) compliance changes.







Similar content being viewed by others
Abbreviations
- LDV:
-
Laser Doppler velocimetry
- MR:
-
Magnetic resonance
- Nd:Ylf:
-
Neodymium-doped yttrium lithium fluoride
- PIV:
-
Particle image velocimetry
- PTV:
-
Particle tracking velocimetry
- US:
-
Ultra sound
- A :
-
Area (cm2)
- C :
-
Compliance (mmHg−1)
- dp/dt :
-
Pressure change with respect to time (mmHg s−1)
- η :
-
Dynamic viscosity (Pa s)
- n :
-
Refractive index
- ρ :
-
Density (kg m−3)
- m :
-
Mass (kg)
- p :
-
Pressure (mmHg)
- v :
-
Velocity (cm s−1)
- T :
-
Temperature (°C)
References
Akutsu, T., and A. Matsumoto. Influence of three mechanical bileaflet prosthetic valve designs on the three-dimensional flow field inside a simulated aorta. J. Artif. Organs 13:207–217, 2010.
Akutsu, T., J. Saito, R. Imai, T. Suzuki, and X. D. Cao. Dynamic particle image velocimetry study of the aortic flow field of contemporary mechanical bileaflet prostheses. J. Artif. Organs 11:75–90, 2008.
Bellhouse, B. J., and F. H. Bellhouse. Mechanism of closure of the aortic valve. Nature 217:86–87, 1968.
Belz, G. G. Elastic properties and Windkessel function of the human aorta. Cardiovasc. Drugs Ther. 9(1):73–83, 1995.
Burgmann, S., S. Große, W. Schröder, J. Roggenkamp, S. Jansen, and F. Gräf. A refractive index-matched facility for fluid–structure interaction studies of pulsatile and oscillating flow in elastic vessels of adjustable compliance. Exp. Fluids 47:865–881, 2009.
Fukuda, I., S. Fujimori, K. Daitoku, H. Yanaoka, and T. Inamura. Flow velocity and turbulence in the transverse aorta of a proximally directed aortic cannula: hydrodynamic study in a transparent model. Ann. Thorac. Surg. 87:1866–1871, 2009.
Geoghegan, P. H., N. A. Buchmann, C. J. T. Spence, S. Moore, and M. Jermy. Fabrication of rigid and flexible refractive-index-matched flow phantoms for flow visualisation and optical flow measurements. Exp. Fluids 52(5):1331–1347, 2012.
Glasser, S. P., D. K. Arnett, G. E. McVeigh, S. M. Finkelstein, A. J. Bank, D. J. Morgan, and J. N. Cohn. Vascular compliance and cardiovascular disease: a risk factor or a marker? Am. J. Hypertens. 10:1175–1189, 1997.
Gülan, U., B. Lüthi, M. Holzner, A. Liberzon, A. Tsinober, and W. Kinzelbach. Experimental study of aortic flow in the ascending aorta via particle tracking velocimetry. Exp. Fluids 53(5):1469–1485, 2012.
Gülan, U., B. Lüthi, M. Holzner, A. Liberzon, A. Tsinober, and W. Kinzelbach. Experimental investigation of the influence of the aortic stiffness on hemodynamics in the ascending aorta. IEEE J. Biomed. Health Inform. 18(6):1775–1780, 2014.
Hall, J. E., and A. C. Guyton. Gyton and Hall Textbook of Medical Physiology. Philadelphia: W. B. Saunders Company, 2000.
Hirata, K., F. Triposkiadis, E. Sparks, J. Bowen, C. F. Wooley, and H. Boudoulas. The Marfan syndrome: abnormal aortic elastic properties. J. Am. Coll. Cardiol. 18:57–63, 1991.
Kelly, R. P., R. Tunin, and D. A. Kass. Effect of reduced aortic compliance on cardiac efficiency and contractile function of in situ canine left ventricle. Circ. Res. 71:490–502, 1992.
Keshavarz-Motamed, Z., E. R. Edelman, P. K. Motamed, J. Garcia, J. N. Dahdah, and L. Kadem. The role of aortic compliance in determination of coarctation severity: lumped parameter modeling, in vitro study and clinical evaluation. J. Biomech. 48(16):4229–4237, 2015.
Keshavarz-Motamed, Z., J. Garcia, N. Maftoon, E. Bedard, P. Chetaille, and L. Kadem. A new approach for the evaluation of the severity of coarctation of the aorta using Doppler velocity index and effective orifice area: in vitro validation and clinical implications. J. Biomech. 45(7):1239–1245, 2012.
Laogun, A. A., and R. G. Gosling. In vivo arterial compliance in man. Clin. Phys. Physiol. Meas. 3:201–2012, 1982.
Liepsch, D., S. Moravec, and R. Baumgart. Some flow visualization and laser-Doppler-velocity measurements in a true-to-scale elastic model of a human aortic arch, a new model technique. Biorheology 29(5–6):563–580, 1992.
Lund-Johansen, P. The hemodynamics of the aging cardiovascular system. J. Cardiovasc. Pharmacol. 12:20–30, 1988.
Madhavan, S., W. L. Ooi, H. Cohen, and M. H. Alderman. Relation of pulse pressure and blood pressure reduction to the incidence of myocardial infarction. Hypertension 23:395–401, 1994.
Nguyen, T. T., R. Mongrain, J. Brunette, J. C. Tardif, and O. F. Bertrand. Method for matching the refractive index and kinematic viscosity of a blood analog for flow visualization in hydraulic cardiovascular models. J. Biomech. Eng. 126:529–536, 2004.
O’Rourke, M. F., A. P. Avolio, and W. W. Nichols. Left ventricular-systemic arterial coupling in humans and strategies to improve coupling in disease states. In: Ventricular-Vascular Coupling, edited by F. C. P. Yin. New York: Springer, 1987, pp. 1–19.
O’Rourke, M. F., J. A. Staessen, C. Vlachopoulos, D. Duprez, and G. E. Plante. Clinical applications of arterial stiffness; definitions and reference values. Am. J. Hypertens. 15:426–444, 2002.
Pielhop, K., C. Schmidt, S. Zholtovski, M. Klaas, and W. Schröder. Experimental investigation of the flow field in an elastic 180° curved vessel. Exp. Fluids 55:1816, 2014.
Raffel, M., C. S. Willert, S. Wereley, and J. Kompenhans. Particle Image Velocimetry: A practical guide. Berlin: Springer, 2007.
Stoiber, M., T. Schlöglhofer, P. Aigner, C. Grasl, and H. Schima. An alternative method to create highly transparent hollow models for flow visualization. Int. J. Artif. Organs 36(2):131–134, 2013.
Yip, R., R. Mongrain, A. Ranga, J. Brunette, and R. Cartier. Development of anatomically correct mock-ups of the aorta for PIV investigations. In: Proceedings of the Canadian Engineering Education Association, 2004.
Conflict of interest
Martin Büsen, Christian Arenz, Michael Neidlin, Sam Liao, Thomas Schmitz-Rode, Ulrich Steinseifer and Simon Sonntag declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editors Hwa Liang Leo and Ajit P. Yoganathan oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Büsen, M., Arenz, C., Neidlin, M. et al. Development of an In Vitro PIV Setup for Preliminary Investigation of the Effects of Aortic Compliance on Flow Patterns and Hemodynamics. Cardiovasc Eng Tech 8, 368–377 (2017). https://doi.org/10.1007/s13239-017-0309-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13239-017-0309-y