Abstract
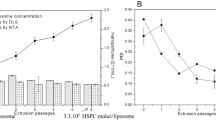
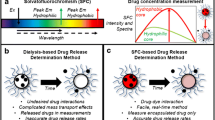
The characterization of encapsulation efficiency and in vitro drug release from nanoparticle-based formulations often requires the separation of nanoparticles from unencapsulated drug. Inefficient separation of nanoparticles from the medium in which they are dispersed can lead to inaccurate estimates of encapsulation efficiency and drug release. This study establishes dynamic light scattering as a simple method for substantiation of the effectiveness of the separation process. Colistin-loaded liposomes, as an exemplar nanosized delivery particle, were diluted to construct a calibration curve relating the amount of light scattering to liposome concentration. Dynamic light scattering revealed that, in the case of ultracentrifugation and centrifugal ultrafiltration, approximately 2.9% of the total liposomes remained in supernatants or filtrates, respectively. In comparison, filtrates obtained using pressure ultrafiltration contained less than 0.002% of the total liposomes from the formulation. Subsequent release studies using dialysis misleadingly implied a slow release of colistin over >48 h. In contrast, pressure ultrafiltration revealed immediate equilibration to the equilibrium distribution of colistin between the liposome and aqueous phases upon dilution. Pressure ultrafiltration is therefore recommended as the optimal method of choice for studying release kinetics of drug from nanomedicine carriers.








Similar content being viewed by others
References
Washington C. Drug release from microdisperse systems: a critical review. Int J Pharm. 1990;58:1–12.
Herman EH, Rahman A, Ferrans VJ, Vick JA, Schein PS. Prevention of chronic doxorubicin cardiotoxicity in beagles by liposomal encapsulation. Cancer Res. 1983;43:5427–32.
Barenholz Y. Relevancy of drug loading to liposomal formulation therapeutic efficacy. J Liposome Res. 2003;13:1–8.
Washington C, Koosha F. Drug release from microparticles: denconvolution of measurement errors. Int J Pharm. 1990;59:79–82.
Magenheim B, Levy MY, Benita S. A new in vitro rechnique for the evaluation of drug release profile from colloidal carriers—ultrafiltration technique at low pressure. Int J Pharm. 1993;94:115–23.
Ricci M, Giovagnoli S, Blasi P, Schoubben A, Perioli L, Rossi C. Development of liposomal capreomycin sulfate formulations: effects of formulation variables on peptide encapsulation. Int J Pharm. 2006;311:172–81.
Zheng Y, Wu Y, Yang W, Wang C, Fu S, Shen X. Preparation, characterization, and drug release in vitro of chitosan-glycyrrhetic acid nanoparticles. J Pharm Sci. 2006;95:181–91.
Wang D, Kong L, Wang J, He X, Li X, Xiao Y. Polymyxin E sulfate-loaded liposome for intravenous use: preparation, lyophilization, and toxicity assessment in vivo. PDA J Pharm Sci Technol. 2009;63:159–67.
Santos Magalhaes NS, Fessi H, Puisieux F, Benita S, Seiller M. An in vitro release kinetic examination and comparative evaluation between submicron emulsion and polylactic acid nanocapsules of clofibride. J Microencapsul. 1995;12:195–205.
Ozer AY, Talsma H. Preparation and stability of liposomes containing 5-fluorouracil. Int J Pharm. 1989;55:185–91.
Cui J, Li C, Deng Y, Wang Y, Wang W. Freeze-drying of liposomes using tertiary butyl alcohol/water cosolvent systems. Int J Pharm. 2006;312:131–6.
Boyd BJ. Characterisation of drug release from cubosomes using the pressure ultrafiltration method. Int J Pharm. 2003;260:239–47.
Ammoury N, Fessi H, Devissaguet JP, Puisieux F, Benita S. In vitro release kinetic pattern of indomethacin from poly(d, l-lactide) nanocapsules. J Pharm Sci. 1990;79:763–7.
Henriksen I, Sverre AS, Gro S, Karlsen J. In vitro evaluation of drug release kinetics from liposomes by fractional dialysis. Int J Pharm. 2005;119:231–8.
Johnston MJ, Edwards K, Karlsson G, Cullis PR. Influence of drug-to-lipid ratio on drug release properties and liposome integrity in liposomal doxorubicin formulations. J Liposome Res. 2008;18:145–57.
Chidambaram N, Burgess DJ. A novel in vitro release method for submicron sized dispersed systems. AAPS PharmSci. 1999;1:E11.
Avgoustakis K, Beletsi A, Panagi Z, Klepetsanis P, Karydas AG, Ithakissios DS. PLGA-mPEG nanoparticles of cisplatin: in vitro nanoparticle degradation, in vitro drug release and in vivo drug residence in blood properties. J Control Release. 2002;79:123–35.
Saarinen-Savolainen P, Jarvinen T, Taipale H, Urtti A. Methods for evaluating drug release from liposomes in sink conditions. Int J Pharm. 1997;159:27–33.
Sezer AD, Akbuga J, Bas AL. In vitro evaluation of enrofloxacin-loaded MLV liposomes. Drug Deliv. 2007;14:47–53.
Muthu MS, Singh S. Poly (d, l-lactide) nanosuspensions of risperidone for parenteral delivery: formulation and in-vitro evaluation. Curr Drug Deliv. 2009;6:62–8.
D’Souza SS, DeLuca PP. Methods to assess in vitro drug release from injectable polymeric particulate systems. Pharm Res. 2006;23:460–74.
Rosenblatt KM, Douroumis D, Bunjes H. Drug release from differently structured monoolein/poloxamer nanodispersions studied with differential pulse polarography and ultrafiltration at low pressure. J Pharm Sci. 2007;96:1564–75.
Nation RL, Li J. Colistin in the 21st century. Curr Opin Infect Dis. 2009;22:535–43.
Falagas ME, Kasiakou SK. Colistin: the revival of polymyxins for the management of multidrug-resistant Gram-negative bacterial infections. Clin Infect Dis. 2005;40:1333–41.
Wallace SJ, Li J, Nation RL, Prankerd RJ, Velkov T, Boyd BJ. Self-assembly behavior of colistin and its prodrug colistin methanesulfonate: implications for solution stability and solubilization. J Phys Chem B. 2010;114:4836–40.
Bangham AD. Membrane models with phospholipids. Prog Biophys Mol Biol. 1968;18:29–95.
Gales AC, Reis AO, Jones RN. Contemporary assessment of antimicrobial susceptibility testing methods for polymyxin B and colistin: review of available interpretative criteria and quality control guidelines. J Clin Microbiol. 2001;39:183–90.
Lide DR. Handbook of chemistry and physics. 76th ed. New York: CRC Press; 1995.
Wallace SJ, Li J, Rayner CR, Coulthard K, Nation RL. Stability of colistin methanesulfonate in pharmaceutical products and solutions for administration to patients. Antimicrob Agents Chemother. 2008;52:3047–51.
Lasch J. Isothermic microcalorimetry. In: Torchillin VP, Weissig V, editors. Liposomes: a practical approach. 2nd ed. Oxford, UK: Oxford University Press; 2003.
New RRC. Liposomes: a practical approach. Oxford: IRL Press; 1990.
Heeremans JL, Gerritsen HR, Meusen SP, Mijnheer FW, Gangaram Panday RS, Prevost R, et al. The preparation of tissue-type plasminogen activator (t-PA) containing liposomes: entrapment efficiency and ultracentrifugation damage. J Drug Target. 1995;3:301–10.
Clausell A, Garcia-Subirats M, Pujol M, Busquets MA, Rabanal F, Cajal Y. Gram-negative outer and inner membrane models: insertion of cyclic cationic lipopeptides. J Phys Chem B. 2007;111:551–63.
Benita S, Levy MY. Submicron emulsions as colloidal drug carriers for intravenous administration: comprehensive physicochemical characterization. J Pharm Sci. 1993;82:1069–79.
Levy MY, Benita S. Drug release from submicronised o/w emulsion: a new in vitro kinetic evaluation model. Int J Pharm. 1990;66:29–37.
Acknowledgments
JL is an Australian National Health and Medical Research Council Senior Research Fellow. This study was supported by Award Number R01AI079330 and Award Number R01AI070896 from the National Institute of Allergy and Infectious Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wallace, S.J., Li, J., Nation, R.L. et al. Drug release from nanomedicines: selection of appropriate encapsulation and release methodology. Drug Deliv. and Transl. Res. 2, 284–292 (2012). https://doi.org/10.1007/s13346-012-0064-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-012-0064-4