Abstract
Very recently, nano zinc oxide (ZnO) has been successfully introduced as a cure activator for the reduction of ZnO level in the rubber industry. The purpose of the present work is to examine the appropriateness of surface-modified nano ZnO in the vulcanization of styrene–butadiene rubber (SBR). In the experimental part, the surface of nano ZnO is modified by stearic acid and bis[3-(triethoxysilyl)propyl]tetrasulfide (Si-69). Si-69-treated nano ZnO causes considerable enhancement in many properties such as maximum rheometric torque, modulus, tensile strength, elongation at break, cross-linking degree of SBR nanocomposite in comparison to conventional ZnO and unmodified or stearic acid-treated nano ZnO. Thermogravimetric analysis (TGA) reveals that Si-69-treated nano ZnO imposes better thermal stability than untreated or stearic acid-treated nano ZnO in the SBR vulcanizates. Morphological study indicates uniform dispersion of Si-69-treated nano ZnO within the SBR matrix and this fact accounts for better mechanical and thermal properties of SBR nanocomposite in the presence of Si-69-modified nano ZnO. This study concludes that Si-69-modified nano ZnO can be effectively applied as cure activator in place of nano ZnO to reduce the ZnO level in SBR compounds. This will lead to both economic advantages and environmental safety in the rubber industry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent times, rubber has been playing an important role in modern civilization. The property attained by a rubber compound greatly depends on the vulcanization process [1]. Vulcanization or cross-linking is a process by which highly soluble plastic compound is converted into insoluble elastic state with improved mechanical properties and increased resistance to temperature variations and solvent action, etc. [1].
In sulfur vulcanization of styrene–butadiene rubber (SBR), the basic ingredients are ZnO, stearic acid, accelerators and sulfur [1]. The ZnO is used as an activator in rubber vulcanization and increases the efficiency of the vulcanizing or cross-linking system. It is assumed that stearic acid accelerates the activity of ZnO which further accelerates the action of the accelerator and sulfur in the vulcanization process.
For industrial use, ZnO is produced at levels of 105 tons per year and about 50–60 % ZnO is used in the rubber industry [2]. In the vulcanization of rubber, the conventional amount of ZnO is 5 phr (parts per hundred parts of rubber). The reduction of ZnO level in the rubber industry is very important from an environmental point of view, as at the end of the products’ life excess ZnO is released into the lithosphere during degradation of rubber [2]. Zinc oxide is also released into the environment through leaching in landfill sites. More importantly, soluble zinc compounds have toxic effect on the aquatic environment [3]. More significantly, the environmental protection agency has classified ZnO as hazardous chemical to the environment and has suggested that its application in rubber compounds should be reduced [1]. To solve this environmental problem, attempts have been made to reduce the ZnO level in rubber vulcanization [4–6].
Unquestionably, there has been a growing interest in the field of polymer nanocomposites because of their special property enhancement [7–9]. Polymer nanocomposites have the ability to achieve remarkable improvement in the mechanical properties due to the addition of small amounts of nanoparticles into the polymer matrix [7].
In recent years, nano ZnO has made very significant contribution to the reduction of ZnO level in rubber compounds [1, 10–13]. Thomas et al. [1] reported the suitability of nano ZnO in comparison to conventional ZnO in the sulfur vulcanization of natural rubber. The results revealed that nano ZnO was able to enhance the mechanical properties of NR. Bhowmick et al. [11] also studied the effect of ZnO nanoparticles on the cure and mechanical properties of natural rubber (NR) and nitrile rubber (NBR). As the authors confirmed, the tensile strength of NR vulcanizate was improved by 80 % in the presence of nano ZnO as a cure activator in comparison to rubber-grade ZnO [11]. Kim et al. [13] studied the effect of nano ZnO on the cure and mechanical properties of unfilled and silica-filled natural rubber (NR)/butadiene rubber (BR) compounds. The authors established that the effect of nano ZnO was more pronounced for silica filled systems than unfilled systems [13]. This was mainly due to the better dispersion of nano ZnO in the presence of dispersing agent used for silica in the rubber matrix [13]. Kim et al. [13] also reported that the optimum amount of nano ZnO in silica-filled natural rubber (NR)/butadiene rubber (BR) compounds is only 1 phr, i.e., nano ZnO leads to five times reduction of the cure activator level in rubber compounds. Very recently, Kalaee et al. [14] described the effect of ZnO nanoparticles on the kinetics of thermal degradation behavior in addition to static and dynamic mechanical properties of ethylene-propylene-diene (EPDM) rubber compounds. It was found that nano ZnO was able to act as both thermal insulator and nano filler for EPDM compounds. Due to the very small particle size and large surface area, nano ZnO is more uniformly dispersed within the rubber matrix in comparison to conventional-grade ZnO, and as a consequence the interfacial interaction between cure activator and rubber matrix also increases in the presence of nano ZnO [11].
Due to the hydrophilic nature of the surface, uniform dispersion of nano ZnO is not possible within the hydrophobic rubber matrix. On the other hand, nanoparticles possess high surface energy [15]. Due to the high surface energy, nano ZnO has the tendency to form aggregate clusters. Thus, surface modification of nano ZnO is necessary to prevent agglomeration of ZnO nanoparticles and also to ensure the perfect dispersion of nano ZnO within the rubber matrix. Undoubtedly, SBR is the most important synthetic rubber, and the major application area for SBR rubber is the production of tires, accounting for almost 70 % of consumption. In the present paper, the surface of sol–gel synthesized nano ZnO is modified by stearic acid and Si-69 to gain better compatibility between inorganic nano ZnO and organic SBR matrix. The main aim of the present article is to prepare SBR nanocomposites with better mechanical and thermal properties in comparison to SBR composites containing either conventional ZnO or unmodified nano ZnO by the proper use of surface-modified nano ZnO. It is noticeable that, 1 phr nano ZnO causes little enhancement in the resulting properties of SBR vulcanizate in comparison to 5 phr conventional ZnO. But, there is an excellent improvement in the mechanical and thermal properties of SBR nanocomposite with 1 phr Si-69-treated nano ZnO in comparison to either 1 phr nano ZnO or 5 phr conventional ZnO. Therefore, 1 phr Si-69-treated nano ZnO is a much more efficient cure activator system than 1 phr nano ZnO and can be applied in the rubber industry to substitute 5 phr conventional ZnO in the SBR formulation. Thus, the present paper is an attempt to develop the rubber industry by suitable use of surface-modified nano ZnO.
Experimental
Materials and physical measurements
Styrene butadiene rubber (SBR 1,502: 23.5 % styrene content, Synthetic and Chemicals Ltd., India), zinc oxide (specific surface area 5–6 m2/g, Merck), stearic acid (Loba Chemie, India), sulfur (Loba Chemie, India), tetrabenzyl thiuram disulfide (TBzTD) (Apollo tyre, Ltd. India), toluene (Merck) and bis[3-(triethoxysilyl)propyl]tetrasulfide (Si-69) (Sigma-Aldrich) were used as received. Nano ZnO was synthesized using the procedure given by Khouzani et al. [16].
X-ray diffraction (XRD) pattern of nano ZnO is recorded on Xpertpro-Panalytical X-ray diffractometer. Field emission scanning electron microscopy (FESEM) images are obtained on a JSM 6700F instrument. Fourier transform infrared (FTIR) spectra (νmax in cm−1) of cross-linked nano ZnO are recorded on a Perkin-Elmer L 120-000A spectrometer (νmax in cm−1) on KBr disks. The cure characteristics of the different stocks are obtained using the Monsanto Rheometer R-100 at 3° arc for 160 °C. The stocks are cured under pressure at 160 °C for optimum cure time (t90), keeping vulcanizates for 24 h at ambient temperature before measuring the modulus at 100 % (M100) elongation, modulus at 300 % (M300) elongation, and tensile strength (T.S.) and elongation at break (E.B. in %) according to ASTM D 412-51 T using dumbbell-shaped test pieces in an Amsler (Sweden) tensile tester. The hardness (shore A) of the vulcanizates is measured by a Hirosima Hardness Tester as per ASTM D 1415-56T. Thermogravimetric analysis (TGA) is carried out to study the thermal behavior of SBR vulcanizates. TGA scans are performed using a TA instrument (Q 5000) under nitrogen flow from 20 to 800 °C with a heating rate of 10 °C/min. The degree of cross-linking is reciprocal with swelling index [17]. Swelling index is measured from equilibrium swelling experiment by allowing the small piece of vulcanizates to swell for 7 days in toluene [17]. Swelling index is determined using the following equation [18]:
where X is the weight of the sample piece after swelling and Y is the weight of the sample piece before swelling.
Synthesis of ZnO nanoparticles
Nano ZnO is prepared via the procedure given by Khouzani et al. [16]. In a typical experiment, 1 mmol Zn(NO3)2, 6H2O and citric acid (CA) (2 mmol) is dissolved in 100 ml deionized water, and ethylene glycol (EG) (EG:CA = 2:1 mol ratio) is then added to form a sol at 50 °C for 1 h. A white solution is obtained and further heated at 80 °C for 1 h to remove excess water. During continued heating at 150 °C for 1 h, the solution becomes more and more viscous and finally a xerogel. To complete drying, the xerogel is placed at 250 °C for 1 h. The resulting powder is a precursor. In the furnace, we heat treated the precursor at 600 °C for 2 h in air, in a ceramic boat, and then cooled it to reach room temperature in a desiccator.
Surface modification of ZnO nanoparticles
The surface of nano ZnO is modified by two different surface modifiers, stearic acid and Si-69. Surface modification of nano ZnO by stearic acid is done by a procedure by Mishra et al. [19]. In this procedure, stearic acid and nano ZnO are separately dissolved in toluene. In the next step, the dispersed solution of nano ZnO is added dropwise for 2 h to the stearic acid solution under constant stirring. Then, the reaction mixture is allowed to settle down. In the last step, the mixture is filtered and washed with toluene followed by methanol to remove unreacted stearic acid. The resulting precipitate is dried in an oven at 80 °C for 12 h until a constant weight is achieved.
Again, surface modification of nano ZnO is carried out using Si-69 as a chemical surface modifier via a procedure by Sombatsompop et al. [20]. At first, Si-69 is mixed with ethanol and the mixture is stirred for 30 min. Then, nano ZnO is mixed with the solution of Si-69 and stirring is continued for 15 min. Then, the Si-69-treated nano ZnO is dried for 12 h at 100 °C to achieve a constant weight. The mechanism of surface modification of nano ZnO by Si-69 is represented in Scheme 1.
Preparation of SBR composites
SBR is masticated in a two-roll mixing mill, and then conventional ZnO and stearic acid are added and again masticated. After that, the accelerator and sulfur are incorporated into the rubber matrix and the mixing is done for about 10 min. The total concentration of the accelerator is fixed at 9 milli mole per 100 g of rubber. The same procedure is repeated to prepare SBR nanocomposites containing unmodified and surface-treated nano ZnO. For the preparation of SBR/Si-69/N ZnO (SM), nano ZnO and Si-69 are simply added to the SBR matrix separately. Then, the mixing procedure is similar to that of the other vulcanizates. The mixing composition of different ingredients is presented in Table 1.
Results and discussion
X-Ray diffraction (XRD)
The XRD pattern of synthesized nano ZnO is recorded in Fig. 1. The detected XRD pattern is found to be similar with that in an earlier report [16]. The diffraction peaks of (100), (002) and (101) at 2θ = 32o, 34.5o and 36.4o are also labeled in Fig. 1.
The average crystallite size is measured from XRD pattern using the well-known Scherrer equation. The Scherrer equation is given as:
where Cs is the average crystallite size, K is a constant of nearly about unity, λ is the wavelength of X-ray (0.154 nm for the CuKα), B is the integral half-width and θ is the Bragg angle.
The average crystallite size is found to be 24.7 nm for the synthesized nano ZnO. The Scherrer equation [21, 22] is commonly used to determine the crystallite size of the nanoparticle.
Field emission scanning electron microscopy (FESEM)
The FESEM image of ZnO nanoparticles is shown in Fig. 2. The particle size of ZnO nanoparticle varies from 40 to 90 nm.
Characterization of surface-modified ZnO nanoparticle
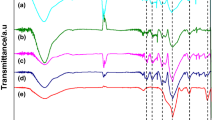
In the present work, the FTIR technique is used to characterize surface-modified ZnO nanoparticles. The FTIR spectra of unmodified and modified nano ZnO are represented in Fig. 3. Figure 3a, b represents the IR spectra of untreated and stearic acid-modified nano ZnO. In Fig. 3b, the sharp peaks at about 2,916 and 2,850 cm−1 specify the presence of long aliphatic chains bonded to the surface of nano ZnO particle [23]. More importantly, the characteristic peaks of the COOH group at 1,713 cm−1 disappear, but the sharp peaks at about 1,455 and 1,538 cm−1 represent the symmetric and antisymmetric carboxylate ion COO− stretching modes [23].
Figure 3c shows the IR spectrum of Si-69-treated nano ZnO. The peaks at about 2,977 and 2,926 cm−1 represent the existence of methylene and methyl group, respectively [24]. Furthermore, the appearance of the peak at about 1,090 cm−1 is due to the formation of silicon–oxygen bond present in the Si-69-treated nano ZnO [24]. This resulting peak confirms that chemical reaction occurs between nano ZnO and Si-69 during surface treatment.
Cure characteristics of SBR vulcanizates
Cure parameters of SBR vulcanizates in the presence of conventional ZnO, unmodified and surface-modified nano ZnO are calculated at 160 °C and the results presented in Table 2. The cure characteristics of SBR vulcanizates are explained in terms of maximum rheometric torque (R∞), optimum cure time (t90), scorch time (t2) and cure rate index (CRI).
The value of maximum rheometric torque (R∞) indicates the level of interaction of the rubber chain with nano ZnO. From the result, it becomes quite clear that the value of R∞ for SBR nanocomposite containing 1 phr unmodified nano ZnO is almost similar to that of the SBR composite containing 5 phr conventional ZnO. For SBR nanocomposites, the value of R∞ increases slightly with incorporation of stearic acid-modified nano ZnO within the rubber matrix in comparison to unmodified nano ZnO. This is due to uniform dispersion of nano ZnO within the rubber phase in the presence of stearic acid as surface modifier. More significantly, it is observed that Si-69-treated nano ZnO has substantial ability to enhance the R∞ value of the SBR nanocomposite. This is mainly attributed to strong chemical interaction between Si-69-treated nano ZnO and SBR chain as represented in Scheme 2. However, there is no significant enhancement in the R∞ value when unmodified nano ZnO and Si-69 are simply added to the SBR matrix.
The rheometric curve of SBR nanocomposites containing unmodified and surface-modified nano ZnO are represented in Fig. 4. The CRI value denotes the rate of the curing process. A greater value of CRI indicates a faster curing process. Stearic acid modification of nano ZnO has no significant effect on the CRI value of SBR nanocomposites. However, in the presence of Si-69-treated nano ZnO the value of optimum cure time (t90) slightly increases and the value of CRI slightly decreases. This may be due to the tendency of Si-69 to prevent the cross-linking reaction [25]. Actually during the mastication process, chemical bond formation occurs between Si-69 and reactive sites of rubber as represented in Scheme 2 [20]. As a result, the chance to form sulfur cross-linking within the rubber matrix decreases. Additionally, the large triethoxysilylpropyl group of Si-69 produces steric hindrance during cross-linking of rubber in the presence of Si-69-treated nano ZnO as cure activator [20]. Both the reasons are responsible for the slight increase in t90 value, i.e., small decrease of CRI value of SBR/Si-69/N ZnO.
Mechanical properties of SBR vulcanizates
The mechanical properties of rubber composites are the most important and deciding parameters for their industrial application. The variation of the mechanical properties of SBR composites are shown in Table 2. The modulus value at 100 % elongation (M100) for SBR/N ZnO is slightly greater than that of SBR/C ZnO. On the other hand, the value of M100 shows an improvement by 8.44 % in the case of SBR/SA/N ZnO containing stearic acid-modified nano ZnO in comparison to SBR/N ZnO containing unmodified nano ZnO. But, there is a significant improvement in the modulus value for SBR nanocomposite containing Si-69-treated nano ZnO, and the value of modulus at 100 % elongation for SBR/Si-69/N ZnO is about 27.12 % greater in comparison to SBR/C ZnO. This is due to the formation of chemical bonding (C–S linkage) between Si-69-modified nano ZnO and rubber during the mastication process as represented in Scheme 2. As a consequence, Si-69-modified nano ZnO has an ability to interact far better with the rubber matrix than both unmodified and stearic acid-modified nano ZnO.
Again, Si-69-treated nano ZnO causes a greater amount of reinforcement in the value of tensile strength in comparison to unmodified or stearic acid-treated nano ZnO. 1 phr nano ZnO results in little enhancement in the value of tensile strength. However, the tensile strength value shows a remarkable increment by 66.92 % for SBR/Si-69/N ZnO in comparison to SBR/C ZnO. The increase in the tensile strength is due to uniform dispersion of Si-69-treated nano ZnO within the rubber matrix. As a result the interaction with the rubber matrix is greater in the presence of Si-69-treated nano ZnO. In a very similar way, the value of elongation at break is also greater for SBR/Si-69/N ZnO than other SBR nanocomposites. However, the value of tensile strength of SBR/Si-69/N ZnO (SM) is almost similar to that of SBR/N ZnO. Thus, it is noticeable that Si-69-modified nano ZnO is much more effective in enhancing the mechanical properties of SBR vulcanizate in comparison to the simple addition of nano ZnO and Si-69 into the rubber matrix. The above results clearly concludes that Si-69-treated nano ZnO is a much more effective cure activator in comparison to stearic acid-treated or untreated nano ZnO for the reduction of ZnO level in SBR compounds.
Degree of cross-linking from swelling studies
The degree of cross-linking values in the rubber matrix of various SBR nanocomposites are calculated from the swelling index and shown in Table 2. The value of cross-linking degree is higher for SBR/Si-69/N ZnO than SBR/N ZnO or SBR/SA/N ZnO. Hence, the value of cross-linking degree clearly indicates better dispersion of nano ZnO for SBR/Si-69/N ZnO in comparison to other two vulcanizates. Moreover, the variation in the degree of cross-linking value is found to be in good agreement with the trend noted in maximum rheometric torque and modulus value in an earlier part of the paper.
Morphology of nanocomposites
The phase morphology of different SBR nanocomposites is also studied to evaluate the extent of dispersion of untreated and surface-treated nano ZnO within the rubber matrix. The SEM images of SBR nanocomposites are shown in Fig. 5a, b and c. The black phase indicates the rubber matrix and the white dot the agglomerated ZnO nanoparticle. In SEM image of SBR/N ZnO (Fig. 5a), some agglomeration of ZnO nanoparticle is observed as a white dot. Figure 5b indicates that nearly uniform dispersion of nano ZnO occurs throughout the rubber matrix in the case of SBR/SA/N ZnO and small numbers of white dots are observed in the SEM image 5b. However in the SEM image of SBR/Si-69/N ZnO (Fig. 5c), much more homogeneous dispersion of Si-69-treated nano ZnO occurs in comparison to stearic acid-treated nano ZnO. As a result, Si-69-treated nano ZnO has excellent ability to improve the mechanical properties of SBR nanocomposites.
Variation of elastic Gibbs free energy (∆G) and conformational entropy (∆S) of SBR vulcanizates
The variation of thermodynamic parameters ∆G and ∆S is represented in Table 3. The elastic Gibbs free energy (∆G) is calculated from the Flory–Huggins equation [26],
where R is the universal gas constant, T is the absolute temperature, Vr is the volume fraction of swollen rubber and χ is the rubber solvent interaction parameter.
The volume fraction of a rubber network in the swollen phase Vr is calculated from equilibrium swelling data using Flory–Rehner equation [27],
where W1 is the weight fraction of the solvent, d1 is the density of the solvent, W2 is the weight fraction of the polymer in the swollen specimen and d2 is the density of the polymer. For the SBR–toluene system, χ = 0.32 [28], the molar volume of toluene Vs is 106.2 cm3/mol and the density of is 0.87 g/cm3.
According to statistical theory of rubber elasticity, ∆S is related to ∆G by the following equation: ∆G = −T∆S, where it is assumed that there is no change in the internal energy of the rubber network during stretching. The data represented in Table 3 indicate that the conformational entropy (∆S) of SBR/Si-69/N ZnO is higher compared to SBR vulcanizates containing conventional ZnO and unmodified or stearic acid-treated nano ZnO. The uniform dispersion of nano ZnO within the rubber matrix is responsible for the higher value of ∆S in SBR/Si-69/N ZnO [1]. The value of ∆G indicates that SBR/Si-69/N ZnO has greater elastic behavior compared to other SBR nanocomposites, as ∆G is closely related to the elastic behavior of the material [1]. The better elastic behavior of SBR/Si-69/N ZnO is explained by considering better compatibility between rubber matrix and Si-69-modified nano ZnO [1].
Thermal properties of SBR vulcanizates
Thermal study plays a vital role in explaining the appropriateness of Si-69-treated nano ZnO instead of either conventional ZnO or nano ZnO in the vulcanization of SBR. Thermogravimetric analysis (TGA) and derivative thermogravimetric analysis (DTA) curves of SBR vulcanizates are shown in Fig. 6a, b. The TGA study compares the thermal stability of SBR/Si-69/N ZnO with other SBR vulcanizates. The study indicates that the rapid degradation region starts at a higher temperature for SBR/Si-69/N ZnO compared to SBR/C ZnO, SBR/N ZnO and SBR/SA/N ZnO. This result confirms that Si-69-treated nano ZnO is able to offer greater thermal stability to the SBR matrix in comparison to either conventional ZnO or stearic acid-treated nano ZnO. The enhancement in the thermal stability of SBR/Si-69/N ZnO over SBR/N ZnO and SBR/SA/N ZnO is further established from DTA study. The onset decomposition temperature (Ti) and the temperature at which the rate of decomposition is maximum (Tmax) are calculated from the DTA curve and defined in Table 4. Although there is no obvious variation in the Tmax values, the onset decomposition temperature (Ti) shifted toward much higher temperature for SBR/Si-69/N ZnO in comparison to SBR/N ZnO and SBR/SA/N ZnO. Thus, it is further concluded from DTA study that Si-69-modified nano ZnO imposes greater thermal stability in SBR vulcanizate than unmodified or stearic acid-treated nano ZnO. The uniform dispersion of Si-69-modified nano ZnO not only restricts the thermal motion of the rubber chain within the network structure, but also causes homogeneous heat distribution throughout the matrix to avoid heat concentration [29, 30]. Both the facts account for the enhanced thermal stability of SBR nanocomposite due to incorporation of Si-69-modified nano ZnO into the rubber matrix.
Conclusions
The present paper mainly focuses on utilizing surface-modified nano ZnO for the preparation of SBR nanocomposites with better mechanical and thermal properties at lower level of ZnO. The surface of nano ZnO is modified by stearic acid and Si-69 to achieve better hydrophobicity. A cure study of SBR vulcanizates indicates that the value of R∞ remains almost unchanged when 1 phr unmodified nano ZnO is applied as a cure activator instead of 5 phr conventional ZnO. But, 1 phr Si-69-treated nano ZnO causes substantial enhancement in the R∞ value of SBR nanocomposite in comparison to 5 phr conventional ZnO. The mechanical properties such as tensile strength and modulus of SBR vulcanizate with 1 phr nano ZnO are slightly greater than conventional SBR vulcanizate. But, 1 phr Si-69-treated nano ZnO causes significant improvement in the mechanical properties of SBR nanocomposite in comparison to 5 phr conventional ZnO. This is associated with the chemical bonding between Si-69-modified nano ZnO and SBR chain during the mastication process. As a result, uniform dispersion of Si-69-modified nano ZnO occurs within the SBR matrix which is confirmed from morphological analysis. However, stearic acid-treated nano ZnO is very less effective in comparison to Si-69-treated nano ZnO in improving cure and mechanical properties of SBR vulcanizate. The value of ∆G indicates that SBR/Si-69/N ZnO has greater elastic behavior compared to other SBR nanocomposites. The TGA study clearly confirms that 1 phr Si-69-treated nano ZnO introduces much greater thermal stability to SBR composites than either unmodified or stearic acid-treated nano ZnO.
The complete study concludes that Si-69-treated nano ZnO is a much more effective property promoter than both unmodified and stearic acid-treated nano ZnO for SBR vulcanizates. Thus, application of 1 phr Si-69-modified nano ZnO instead of 1 phr nano ZnO will be a much better choice for rubber researchers for replacing 5 phr conventional ZnO in SBR compounds. Thus, the present study has an important impact from both environmental and industrial point of view.
References
Panampilly, B., Thomas, S.: Nano ZnO as cure activator and reinforcing filler in natural rubber. Polym. Eng. Sci. 53, 1337–1346 (2013)
Das, A., Wang, D.Y., Leuteritz, A., Subramaniam, K., Greenwell, H.C., Wagenknecht, U., Heinrich, G.: Preparation of zinc oxide free, transparent rubber nanocomposites using a layered double hydroxide filler. J. Mater. Chem. 21, 7194–7200 (2011)
Fosmire, G.J.: Zinc toxicity. Am. J. Clin. Nutr. 51, 225–227 (1990)
Heideman, G., Datta, R.N., Noordermeer, J.W.M., Van Baarle, B.: Effect of zinc complexes as activator for sulfur vulcanization in various rubbers. Rubber Chem. Technol. 78, 245–257 (2005)
Heideman, G., Datta, R.N., Noordermeer, J.W.M., Van Baarle, B.: Zinc loaded clay as activator in sulfur vulcanization: a new route for zinc oxide reduction in rubber compounds. Rubber Chem. Technol. 77, 336–355 (2004)
Heideman, G., Datta, R.N., Noordermeer, J.W.M., Van Baarle, B.: Multifunctional additives as zinc-free curatives for sulfur vulcanization. Rubber Chem. Technol. 79, 561–588 (2006)
Jahanmardi, R., Kangarlou, B., Dibazar, A.R.: Effects of organically modified nanoclay on cellular morphology, tensile properties, and dimensional stability of flexible polyurethane foams. J. Nanostruct. Chem. 3, 82 (2013)
Shokrieh, M.M., Saeedi, A., Chitsazzadeh, M.: Mechanical properties of multi-walled carbon nanotube/polyester nanocomposites. J. Nanostruct. Chem. 3, 20 (2013)
Ghadim, M.F., Imani, A., Farzi, G.: Synthesis of PPy–silver nanocomposites via in situ oxidative polymerization. J. Nanostruct. Chem. 4, 101 (2014)
Sahoo, S., Bhowmick, A.K.: Influence of ZnO nanoparticles on the cure characteristics and mechanical properties of carboxylated nitrile rubber. J. Appl. Polym. Sci. 106, 3077–3083 (2007)
Sahoo, S., Maiti, M., Ganguly, A., George, J.J., Bhowmick, A.K.: Effect of zinc oxide nanoparticles as cure activator on the properties of natural rubber and nitrile rubber. J. Appl. Polym. Sci. 105, 2407–2415 (2007)
Jincheng, W., Yuehui, C.: Application of nano-zinc oxide master batch in polybutadiene styrene rubber system. J. Appl. Polym. Sci. 101, 922–930 (2006)
Kim, Il-J, Kim, W.S., Lee, D.H., Kim, W., Bae, J.W.: Effect of nano zinc oxide on the cure characteristics and mechanical properties of the silica-filled natural rubber/butadiene rubber compounds. J. Appl. Polym. Sci. 117, 1535–1543 (2010)
Kalaee, M., Akhlaghi, S., Mazinani, S., Sharif, A., Jarestani, Y.C., Mortezaei, M.: Effect of ZnO nanoparticles on kinetics of thermal degradation and final properties of ethylene-propylene-diene rubber systems. J. Therm. Anal. Calorim. 110, 1407–1414 (2012)
Mallakpour, S., Madani, M.: Use of silane coupling agent for surface modification of zinc oxide as inorganic filler and preparation of poly(amide–imide)/zinc oxide nanocomposite containing phenylalanine moieties. Bull. Mater. Sci. 35, 333–339 (2012)
Khouzani, M.F., Fereshteh, Z., Estarki, M.R.L., Razavi, R.S.: Different morphologies of ZnO nanostructures via polymeric complex sol–gel method: synthesis and characterization. J. Sol-Gel. Sci. Technol. 64, 193–199 (2012)
Kapgate, B.P., Das, C., Das, A., Basu, D., Reuter, U., Heinrich, G.: Effect of sol–gel derived in situ silica on the morphology and mechanical behavior of natural rubber and acrylonitrile butadiene rubber blends. J. Sol-Gel. Sci. Technol. 63, 501–509 (2012)
Mishra, S., Shimpi, N.G.: Mechanical and flame-retarding properties of styrene–butadiene rubber filled with nano-CaCO3 as a filler and linseed oil as an extender. J. Appl. Polym. Sci. 98, 2563–2571 (2005)
Mishra, S., Shimpi, N.G., Mali, A.D.: Investigation of photo-oxidative effect on morphology and degradation of mechanical and physical properties of nano CaCO3 silicone rubber composites. Polym. Adv. Technol. 23, 236–246 (2012)
Thongsang, S., Sombatsompop, N.: Effect of NaOH and Si69 treatments on the properties of fly ash/natural rubber composites. Polym. Compos. 27, 30–40 (2006)
Kar, S., Bhowmick, A.K.: Nanostructured magnesium oxide as cure activator for polychloroprene rubber. J. Nanosci. Nanotechnol. 9, 3144–3153 (2009)
Roy, K., Alam, M.N., Mandal, S.K., Debnath, S.C.: Sol–gel derived nano zinc oxide for the reduction of zinc oxide level in natural rubber compounds. J. Sol-Gel. Sci. Technol. 70, 378–384 (2014)
Badre, C., Pauporté, T., Turmine, M., Lincot, D.: A ZnO nanowire array film with stable highly water-repellent properties. Nanotechnol. 18, 365705 (2007)
Wang, Z., Lu, Y., Liu, J., Dang, Z., Zhang, L., Wang, W.: Preparation of Nano-Zinc Oxide/EPDM Composites with Both Good Thermal Conductivity and Mechanical Properties. J. Appl. Polym. Sci. 119, 1144–1155 (2011)
Rooj, S., Das, A., Thakur, V., Mahaling, R.N., Bhowmick, A.K., Heinrich, G.: Preparation and properties of natural nanocomposites based on natural rubber and naturally occurring halloysite nanotubes. Mater. Des. 31, 2151–2156 (2010)
Usuki, A., Kawasumi, M., Kojima, Y., Okada, A., Kurauchi, T., Kamigaito, O.: Synthesis of nylon 6-clay hybrid. J. Mater. Res. 8, 1179–1184 (1993)
Flory, P.J., Renher, J.J.: Statistical mechanics of cross-linked polymer networks II. swelling. J. Chem. Phys. 11, 521–526 (1943)
Sheehan, C.J., Bisio, A.L.: Polymer/solvent interaction parameters. Rubber Chem. Technol. 39, 149–192 (1966)
Mishra, S., Shimpi, N.G., Patil, U.D.: Effect of nano CaCO3 on thermal properties of styrene butadiene rubber (SBR). J. Polym. Res. 14, 449–459 (2007)
Mishra, S., Shimpi, N.G., Mali, A.D.: Influence of stearic acid treated nano-CaCO3 on the properties of silicone nanocomposites. J. Polym. Res. 18, 1715–1724 (2011)
Acknowledgments
The authors thankfully acknowledge DST-PURSE programme, Government of India, for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under license to BioMed Central Ltd. Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Roy, K., Alam, M.N., Mandal, S.K. et al. Surface modification of sol–gel derived nano zinc oxide (ZnO) and the study of its effect on the properties of styrene–butadiene rubber (SBR) nanocomposites. J Nanostruct Chem 4, 133–142 (2014). https://doi.org/10.1007/s40097-014-0127-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40097-014-0127-9