Abstract
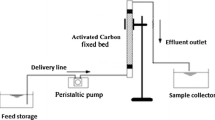
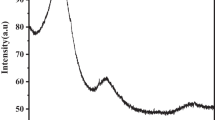
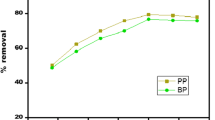
The adsorption of malachite green (MG) and methyl orange (MO) from aqueous solution by waste tyre-derived activated carbon (WTAC) using in batch and fixed-bed column methods was studied. Freundlich model with high correlation coefficient (0.99) described the adsorption data more suitably than other models, indicating multi-layer adsorption of dyes onto heterogeneous WTAC surface. The maximum Langmuir monolayer adsorption capacity was 29.23 g/kg for MG and 13.56 g/kg for MO. The adsorption obeyed pseudo-second order model, following both film-diffusion as well as intra-particle diffusion mechanisms. The thermodynamic parameters indicated spontaneous and endothermic adsorption. Fixed-bed column studies at varying flow rates between 3–5 mL/min for MG and 2–3 mL/min for MO, initial dye concentration (40–50 mg/L), and bed heights (1–2 cm) indicated that breakthrough time and exhaustion time increased with decreasing flow rate, increasing bed height and decreasing initial dyes concentration. The experimental data fitted well with Thomas and Adams–Bohart models with maximum Thomas model adsorption capacity of 71.71 g/kg for MG and 6.86 g/kg for MO. Thus, WTAC proved a promising adsorbent for the removal of MG and MO from aqueous solution.














Similar content being viewed by others
References
Ali I, Khan TA, Asim M (2012) Removal of arsenate from groundwater by electrocoagulation method. Environ Sci Pollut Res 19:1668–1676
Alqaragully MB (2014) Removal of textile dye (maxilon blue and methyl orange) by date stone activated carbon. Int J Adv Res Chem Sci 1:48–59
Annadurai G, Juang R-S, Lee D-J (2002) Use of cellulose-based wastes for adsorption of dyes from aqueous solutions. J Hazard Mater 92:263–274
Bohart GS, Adams EQ (1920) Behavior of charcoal towards chlorine. J Chem Soc 42:523–529
Boyd GE, Adamson AW, Myers LS Jr (1947) The exchange adsorption of ions from aqueous solutions by organic zeolites (II) kinetics. J Am Chem Soc 69:2836–2848
Deng H, Yang L, Tao G, Dai J (2009) Preparation and characterization of activated carbon from cotton stalk by microwave assisted chemical activation: application in methylene blue adsorption from aqueous solution. J Hazard Mater 166:1514–1521
Dubinin MM, Radushkevich LV (1947) The equation of characteristic curve of the activated charcoal. Proc Acad Sci USSR Phys Chem Sect 55:331–337
APHA (2005) Standard methods for the examination of water and wastewater. In: Eaton AD, Franson MAH (eds) American Public Health Association (APHA), Washington, DC
Falaki F, Fakhri A (2014) Study of the adsorption of methyl orange from aqueous solution using nickel oxide nanoparticles: equilibrium and kinetics studies. J Phys Theor Chem IAU Iran 10:117–124
Foo KY, Lee LK, Hameed BH (2013) Preparation of tamarind fruit seed activated carbon by microwave heating for the adsorptive treatment of landfill leachate: a laboratory column evaluation. Bioresour Technol 133:599–605
Freundlich HZ (1906) Uber die adsorption in losungen. Z Phys Chem 57:385–470
Ghaedi M, Golestani Nasab A, Khodadoust S, Rajabi M, Azizian S (2014a) Application of activated carbon as adsorbents for efficient removal of methylene blue: kinetics and equilibrium study. J Ind Eng Chem 20:2317–2324
Ghaedi M, Ghaedi AM, Ansari A, Mahammadi F, Vafaei A (2014b) Artificial neural network and particle swarm optimization for removal of methyl orange by gold nano particles loaded on activated carbon and tamarisk. Spectrochim Acta Part A 132:639–654
Goertzen SL, Theriault KD, Oickel AM, Tarasuk AC, Andreas HA (2010) Standardization of the Boehm titration. Part I. CO2 expulsion and end point determination. Carbon 48:1252–1261
Gong R, Jin YF, Chen J, Liu Z (2006) Enhanced malachite green removal from aqueous solution by citric acid modified rice straw. J Hazard Mater 137:865–870
Gong R, Ye J, Dai W, Yan X, Hu J, Hu X, Li S, H Huang (2013) Adsorptive removal of methyl orange and methylene blue from aqueous solution with finger-citron-residue-based activated carbon. Ind Eng Chem Res 52:14297–14303
Gupta VK, Gupta B, Rastogi A, Agarwal S, Nayak A (2011) A comparative investigation on adsorption performances of mesoporous activated carbon prepared from waste rubber tire and activated carbon for a hazardous azo dye–Acid Blue 113. J Hazard Mater 186(1):891–901
Gupta VK, Ali I, Saleh TA, Nayak A, Agarwal S (2012) Chemical treatment technologies for waste-water recycling—an overview. RSC Adv 2:6380–6388
Gupta VK, Ali I, Saleh TA, Siddiqui MN, Agarwal S (2013) Chromium removal from water by activated carbon developed from waste rubber tyres. Environ Sci Pollut Res 20:1261–1268
Ho YS, Mckay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465
Hou X-X, Deng QF, Ren T-Z, Yuan Z-Y (2013) Adsorption of Cu2+ and methyl orange from aqueous solution by activated carbon of corncob-derived char waste. Environ Sci Pollut Res 20:8521–8553
Iida Y, Kozuka T, Tuziuti T, Yasui K (2004) Sonochemically enhanced adsorption and degradation of methyl orange with activated aluminas. Ultrasonics 42:635–639
Karthikeyan S, Gupta VK, Boopathy R, Titus A, Sekaran G (2012) A new approach for the degradation of high concentration of aromatic amine by heterocatalytic Fenton oxidation: kinetic and spectroscopic studies. J Mol Liq 173:153–163
Khan TA, Singh V (2010) Removal of cadmium(II), lead(II), and chromium(VI) ions from aqueous solution using clay. Toxicol Environ Chem 92:1435–1446
Khan TA, Dahiya S, Ali I (2012) Removal of direct red 81 dye from aqueous solution by native and citric acid modified bamboo sawdust—kinetic study and equilibrium isotherm analyses. G U J Sci 25:59–87
Khan TA, Sharma S, Khan EA, Mukhlif AA (2014) Removal of congo red and basic violet 1 by chir pine (Pinus roxburghii) sawdust, a saw mill waste: batch and column studies. Toxicol Environ Chem 96:555–568
Khan TA, Nazir M, Khan EA (2015a) Magnetically modified multiwalled carbon nanotubes for the adsorption of bismarck brown R and Cd (II) from aqueous solution: batch and column studies. Desal Water Treat. doi:10.1080/19443994.2015.1100553
Khan TA, Nazir M, Khan EA, Riaz U (2015b) Multiwalled carbon nanotube–polyurethane (MWCNT/PU) composite adsorbent for safranin T and Pb (II) removal from aqueous solution: batch and fixed-bed studies. J Mol Liq 212:467–479
Khan TA, Mukhlif AA, Khan EA, Sharma DK (2016a) Isotherm and kinetics modeling of Pb(II) and Cd(II) adsorptive uptake from aqueous solution by chemically modified green algal biomass. Model Earth Syst Environ doi:10.1007/s40808-016-0157-z
Khan TA, Khan EA, Shahjahan (2016b) Adsorptive uptake of basic dyes from aqueous solution by novel brown linseed deoiled cake activated carbon: equilibrium isotherms and dynamics. J Environ Chem Eng 4:3084–3095
Khattri SD, Singh MK (2009) Removal of malachite green from dye wastewater using neem sawdust by adsorption. J Hazard Mater 167:1089–1094
Kumar KV (2007) Optimum sorption isotherm by linear and non-Linear methods for malachite green onto lemon peel. Dyes Pig 74:595–597
Kumar M, Tamilarasan R (2013) Modeling studies for the removal of methylene blue from aqueous solution using Acacia fumosa seed shell activated carbon. J Environ Chem Eng 1:1108–1116
Lagergren S (1898) Zur theorie der sogenannten adsorption gelöster stoffe. K Sven Vetenskaps Akad Handl 4:1–39
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1367
Leung SY, Cheng WH, McKay G (2009) Acid dyes adsorption onto activated carbon from waste tyres. Int J Environ Waste Manag 3:286–296
Li L, Liu S, Zhu T (2010) Application of activated carbon derived from scrap tires for adsorption of rhodamine B. J Environ Sci 22(8):1273–1280
Lopez FA, Centeno TA, Rodriguez O, Alguacil FJ (2013) Preparation and characterisation of activated carbon from the char produced in the thermolysis of granulated scrape tyre. J Air Waste Manag Assoc 63:534–544
Mahamad MN, Zaini MAA, Zakaria ZA (2015) Preparation and characterization of activated carbon from biomass for dye removal. Int Biodeter Biodegr 102:274–280
Malana MA, Amanat T, Qureshi RB, Ahmad HB, (2012) Biosorptive removal of malachite green, methylene blue and methyl orange dyes from aqueous solutions by Ficus bengalensis (banyan) tree leaves. Asian J Chem 24: 3070–3074
Mui ELK, Ko DCK, McKay G (2004) Production of active carbons from waste tyres- a review. Carbon 42:2789–2805
Mui ELK, Chang WH, Valix M, McKay G (2010a) Mesoporous activated carbon from waste tyre rubber for dye removal from effluents. Micropor Mesopor Mater 130(1–3):287–294.
Mui ELK, Chang WH, Valix M, McKay G (2010b) Dye adsorption onto activated carbon from tyre rubber waste using surface coverage analysis. J Colloid Interface Sci 347(2):290–300
Pal J, Deb MK, Deshmukh DK (2013) Removal of methyl orange by activated carbon modified by silver nanoparticles. Appl Water Sci 3:367–374
Porkodi K, Kumar KV (2007) Equilibrium, kinetics and mechanism modeling and simulation of basic and acid dyes sorption onto jute fiber carbon: eosin yellow, malachite green and crystal violet single component systems. J Hazard Mater 143:311–327
Saha P, Chowdhery S, Gupta S, Kumar I, Kumar R (2010) Assessment on the removal of malachite green using tamarind fruit shell as biosorbent. Clean-Soil Air Water 38:437–445.
Saleh TA, Gupta VK (2012) Photo-catalyzed degradation of hazardous dye methyl orange by use of a composite catalyst consisting of multi-walled carbon nanotubes and titanium dioxide. J Colloid Interface Sci 371:101–106
Saleh TA, Al-Saadi AA, Gupta VK (2014) Carbonaceous adsorbent prepared from waste tires: experimental and computational evaluations of organic dye methyl orange. J Mol Liq 191:85–91
Santhi T, Manonmani S, Smitha T, Mahalaksmi K (2009) Adsorption of malachite green from aqueous solution onto a waste aqua cultural shell powders (prawn waste): kinetics study. Rasayan J Chem 2:813–824
Sharma YC, Uma, Upadhyay SN (2011) An economically viable removal of methylene blue by adsorption on activated carbon prepared from rice husk. Can J Chem Eng 82:377–383
Srivastav AK, Roy D (2015) Effects of malachite green (Triarylmethane dye) and Pyceze (Bronopol) on the hematological parameters of a freshwater catfish Heteropneustes fossilis (Bloch). Int J Fish Aqua Stud 2:119–122
Tahir SS, Rauf N (2006) Removal of a cationic dye from aqueous solutions by adsorption onto bentonite clay. Chemosphere 63:1842–1848
Temkin MJ, Pyzhev V (1940) Kinetics of ammonia synthesis on promoted iron catalysis. Acta Physiochimica USSR 12:327–357
Teng H, Lin Y-C, Hsu L-Y (2000) Production of activated carbon from pyrolysis of waste tires impregnated with potassium hydroxide. Air Waste Manag Assoc 50: 1940–1946
Thomas HC (1944) Heterogeneous ion exchange in a flowing system. J Am Chem Soc 66:1466–1664
Urban waste profile, ENVIS newsletter (2010) Central Pollution Control Board, New-Delhi, India. pp 12
Weber WJ, Morris JC (1964) In: Eckenfelder WW (ed) Advances in water pollution research. Pergamon Press, Oxford
Zhang J, Li Y, Zhang C, Jing Y (2008) Adsorption of malachite green from aqueous solution onto carbon prepared from Arundo donax root. J Hazard Mater 150:774–782
Acknowledgements
The author (RR) thanks University Grants Commission, New Delhi for Non-NET fellowship.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Khan, T.A., Rahman, R. & Khan, E.A. Adsorption of malachite green and methyl orange onto waste tyre activated carbon using batch and fixed-bed techniques: isotherm and kinetics modeling. Model. Earth Syst. Environ. 3, 38 (2017). https://doi.org/10.1007/s40808-017-0284-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40808-017-0284-1