Abstract
During acid leaching of bauxite residue (red mud), the increase in dissolution of rare-earth elements (REEs) is associated with an increase in iron dissolution, which poses problems in the downstream processing. Therefore, it would be beneficial to remove iron from bauxite residue by smelting reduction. The slag generated in the smelting reduction process could then be further processed for recovery of REEs. Smelting experiments were carried out at temperatures between 1500 and 1600 °C. Wollastonite (CaSiO3) was used as a flux and graphite as a reducing agent. The addition of wollastonite decreases the slag melting temperature and the viscosity, facilitating slag-metal separation, whereas a graphite content higher than the optimum level alters the slag chemistry and hinders the slag-metal separation. The optimum conditions were found to be for heating at 1500 °C: 20 wt% of wollastonite and 5 wt% of graphite. More than 85 wt% of the iron was separated from the slag in the form of a nugget. A further 10 wt% of the iron could be extracted from the slag by subsequent grinding and magnetic separation. The slag obtained after iron removal was treated with HCl, HNO3, and H2SO4 acids to extract REEs. Room-temperature leaching was found to be not beneficial for REEs extraction. High-temperature leaching enhanced the recovery of REEs. More than 95 % of scandium, >70 % of REEs, and about 70 % of titanium could be leached at 90 °C. The selectivity of REEs over iron during slag leaching was clearly improved.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
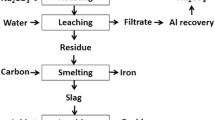
Bauxite is the primary ore for aluminum extraction. It is treated with sodium hydroxide at above 200 °C to extract alumina in the Bayer’s process. Iron, together with impurities that are insoluble in the caustic solution, will be removed by clarification. The residue generated after clarification is known as bauxite residue (or red mud). About 1.5–2.5 tons of bauxite residue is generated per ton of alumina produced. It is stored mainly in large storage ponds, with potentially environmentally harmful effects. There is no bulk application of bauxite residue except the use of small amounts in cement and ceramic production [1]. On the other hand, some of the bauxite residues are rich in REEs [2]. Extraction of REEs and of scandium in particular from bauxite residue can be economically feasible [3].
Bauxite deposits on carbonate rocks are known as karst bauxites. These deposits account for around 14 % of the total bauxite reserves. Karst bauxite ores are rich in REEs [4]. These REEs end up in the bauxite residue during the Bayer’s process. The REEs can be recovered from the bauxite residue by direct acid leaching [3, 5–7]. The yield of extraction of REEs is low, but it can be improved at high acid concentrations, and the effect is more pronounced for HCl compared to H2SO4 or HNO3. However, these strongly acidic conditions will also bring large amounts of iron into solution [6]. Iron dissolution is not beneficial as it is difficult to separate iron from the REEs and especially from scandium, requiring a large amount of reagents during the further processing such as solvent extraction [7]. Therefore, it was proposed to remove iron by smelting reduction so that the REEs can be concentrated in the slag phase, which then subsequently can be leached with the help of acids for the sake of extracting REEs [2, 8–10].
Iron removal studies from bauxite residue can be classified into two major approaches: (1) solid-state reduction and (2) smelting. In a solid-state reduction process, bauxite residue is reduced with a solid or gaseous reductant, resulting in the formation of Fe3O4 or metallic iron which can be used for the metal production with or without prior magnetic separation [11–13]. So far, no solid-state reduction process has been commercialized yet [14, 15], because of specific problems associated with the bauxite residue such as low iron content, high alkali content, fineness of the particles, moisture etc. In the smelting approach, bauxite residue is treated in a blast furnace with prior sintering in the presence of a reducing agent in order to reduce the iron oxides, generating pig iron and slag. Low concentrations of iron and high concentrations of sodium are the major drawbacks for utilizing bauxite residue in the blast furnace [2]. Therefore, use of alternative smelting methods should be considered for the production of iron from bauxite residue. They include the Corex, Finex, Hismelt, Romelt, AusIron, and electric arc furnace (EAF) processes [16]. So far, two smelting processes were tested on a large scale for bauxite residue smelting. These are the Romelt process [17] and the EAF smelting process [8, 18, 19]. The Moscow Institute of Steel and Alloys (MISA), together with NALCO and RSIL (India), studied processing of bauxite residue by the Romelt process [17]. The main disadvantage of this process is the high energy consumption and the poor quality of the produced pig iron (high sulfur content) [16]. In the EAF process, a mixture of bauxite residue and coal was smelted in an EAF at 1600–1700 °C to form an iron alloy with more than 90 % extraction of iron [18, 19]. The slag generated after smelting can be used for the production of slag wool [18] or building materials [20], as well as for the extraction of titanium [8, 19, 21], other non-ferrous metals or REEs [2, 8–10]. REEs from the slag were leached with the help of a sulfuric acid solution [8–10, 22].
High temperatures or large amounts of fluxes are required for smelting of bauxite residue due to the high alumina content. Both high temperature and high amount of flux increase the energy consumption during smelting. In addition, the high amount of flux increases the acid consumption during leaching. Therefore, in this work, the amount of flux was optimized with respect to slag-metal separation. The carbon content was also optimized to obtain a clear slag-metal separation. The slag generated after smelting was leached with different acids to study the recovery of the different elements.
Experiments and Methods
The bauxite residue used in this work was provided by the Aluminum of Greece. It is generated predominantly from Greek (karst) bauxite ore. Chemical analysis of the major elements was performed using wavelength-dispersive X-ray fluorescence spectroscopy (WDXRF, Panalytical PW2400), whereas that of the minor elements was performed by complete dissolution of the bauxite residue by alkali fusion and acid digestion in a 1:1 (v/v) HCl solution, followed by Inductively Coupled Plasma Mass Spectrometry (ICP-MS, Thermo Electron X Series) analysis. Thermodynamic calculations, based on the chemical analysis, were performed using the FactSage 6.4 software [23]. The slag melting point, effect of different fluxes on slag melting point, and phase equilibria at different temperatures were studied. All the major slag forming oxides (Al2O3, CaO, SiO2, and TiO2) were considered in the calculations. Na2O was not considered in the calculations because of its low amount in the sample and its volatile behavior during smelting. FeOx was not used in the calculations as it will be reduced to metallic iron during smelting. The wt% of carbon and fluxes showed in the FactSage studies and smelting experiments are expressed with respect to the weight of the bauxite residue.
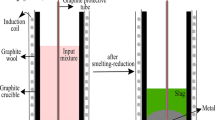
The bauxite residue sample was mixed with high purity (>99.5 %) graphite powder (Superior Graphite Co.) and wollastonite (Sibelco Specialty Minerals) (CaO—51.2 % and SiO2—46.4 %) using a mortar and pestle. Graphite was used instead of other commercial reductants to make this scientific study uncomplicated as the graphite does not contain any volatiles and ash. Handmade pellets were prepared and dried at 105 °C for 12 h. A graphite crucible was used to contain the pellets. The smelting reduction experiments were carried out in a high-temperature vertical alumina tube furnace (Gero HTRV 100–250/18, with MoSi2 heating elements). High purity argon gas (99.999 %) with a flow rate of 0.4 L min−1 was used to control the atmosphere in the furnace. Pellets were heated to 1500–1600 °C with a heating rate of 5 °C min−1 and kept at that preset temperature for 1 h. After heating, the sample was cooled to room temperature at a cooling rate of 4 °C min−1. The reduced samples were then embedded in epoxy resin and polished with SiC abrasive paper down to 1200 grit size followed by polishing with diamond paste (6, 3 and 1 μm) on a cloth disk. Then, the samples were coated with platinum and analyzed with scanning electron microscope (SEM–EDX, Philips XL30). The metal produced in the smelting experiment was analyzed with WDXRF, Panalytical PW2400 for its chemical composition, and the carbon and sulfur contents were measured by LECO combustion analysis (type CS-444, based on infrared absorption).
The slag was crushed into small pieces (<4 mm) with a jaw crusher (Retsch BB100), followed by grinding in a centrifugal mill (Retsch ZM100) to reduce the particle size to <80 μm. Small iron particles in the slag sample were removed by a magnet after grinding. The chemical analysis procedure for the slag is the same as for the bauxite residue. Room-temperature leaching experiments were carried out in sealed 50-ml polyethylene bottles by constant agitation using a laboratory shaker (Gerhardt Laboshake) at 160 rpm and 25 °C. High-temperature leaching experiments were carried out in a 500-mL glass reactor fitted with a reflux condenser and placed on a temperature-controlled ceramic hot plate with a magnetic stirring system. Analytical reagent grade nitric acid (65 %) (Chem-lab), sulfuric acid (95–97 %) (Sigma–Aldrich), and hydrochloric acid (37 %) (Fisher Scientific) were used in the present study. The leach solution sample was filtered using a syringe filter (pore size of 0.45 µm) and diluted with deionised water (Milli-Q, resistance 18.2 MΩ cm) for ICP-MS analysis.
Results and Discussion
The chemical analysis of the bauxite residue used in this study is shown in Tables 1 and 2. Table 1 shows that the bauxite residue is rich in iron oxide and alumina. Table 2 shows that the total REE content in the bauxite residue is about 0.1 %. It also shows the total REE content of the slag generated after smelting experiments for the sake of comparison. Borra et al. found that the bauxite residue used in this study was a very fine material with d90 <10 µm containing small agglomerates [6]. They also found that it contains different phases like hematite, goethite, gibbsite, diaspore, calcite, and cancrinite from XRD analysis (Fig. 1).
XRD pattern of the bauxite residue sample [6]
Thermodynamic Calculations
Figure 2 shows the effect of temperature on the phase equilibria of the slag without any addition of flux. It shows that with an increase in the temperature, the amount of liquid phase is increasing. A temperature of about 1600 °C is required to melt the slag completely. The slag should be liquid and fluid (less viscous) for good slag-metal separation. The presence of any solid phase in the slag drastically decreases the slag fluidity.
The effect of different fluxes was studied on the phase equilibria of the slag. The wt% of all the components in the slag is not equal to 100 % due to the fact that Fe was removed and fluxes are added. Figure 3 shows the effect of CaO on the phase equilibria at 1500 °C. In this Figure, it can be observed that there is no complete liquid formation up to 40 wt% of lime. On the other hand, 15 wt% of SiO2 can completely dissolve the solid phases (Fig. 4). However, although 15 wt% of SiO2 decreases the slag basicity, it also increases the slag viscosity. Therefore, CaSiO3 was investigated as a flux, which maintains the slag basicity around one. Figure 5 shows the effect of the amount of CaSiO3 on the phase equilibria. It can be concluded from this Figure that 15 wt% of CaSiO3 is sufficient to make the slag liquid at 1500 °C.
Both CaF2 and B2O3 were not considered as fluxes. CaF2 is toxic because of fluorides emission, corrodes the refractories [24], and also can form HF during leaching with strong acids. B2O3 corrodes the refractories and a fraction of it also reduces to metal during smelting [25]. Furthermore, FactSage showed that the amount of B2O3 required for slag melting is larger than 10 wt %, which would make such an addition very expensive compared to CaSiO3 flux.
Smelting Studies
The requirement of carbon for the reduction of iron oxide was calculated based on the stoichiometric equation (Eq. 1).
The initial experiment was carried out at 1500 and 1600 °C with 100 % excess of stoichiometric amount of carbon (10 wt% of the bauxite residue) and without flux addition. Excess carbon was used because there is also some CO formation taking place at high temperatures during the reduction. No clear slag-metal separation was observed in the samples. Partial segregation of the metal was observed at the bottom of the sample due to the high density of the metal compared to that of the slag phase. SEM–EDX analysis (Fig. 6) of the sample smelted at 1600 °C shows that some of the SiO2 and most of the TiO2 are reduced to the metal phase. Therefore, further experiments were carried out with a decreased amount of graphite addition. Figure 7 shows the SEM images of the sample containing 20 wt% wollastonite and 40 % excess of stoichiometric carbon (7 wt%) and smelted at 1500 °C. Wollastonite enhanced the metal separation but not up to the extent required. It was observed that the iron metal phase in the sample was locked by a titanium oxycarbide phase, which is prohibiting iron to separate from the slag phase. Logomerac also faced the tapping problem due to the reduction of TiO2 during smelting [8]. Therefore, the graphite content was even further decreased to the exact stoichiometric amount. A clear slag-metal separation (Fig. 8) was now observed at 1500 °C with 20 wt% wollastonite and stoichiometric carbon (5 wt%). No iron oxides were observed in the slag phase by SEM–EDX analysis, which means that the amount of added carbon was sufficient for a complete iron oxide reduction. However, the carbon requirement will be more than stoichiometric as there will be some CO formation on the one hand, and some amount of carbon will be dissolved in the metal phase on the other hand. This deficient carbon is presumed to be extracted from the graphite crucible. 5 wt% of graphite was chosen as the optimized amount at these experimental conditions, although it may vary depending on the heating rate used during smelting and reaction between the carbon crucible or refractory. An experiment was also conducted at 1600 °C with 20 wt% wollastonite and stoichiometric carbon (5 wt%). But the slag-metal separation was low. It is due to the reaction between sample and graphite crucible at high temperature, which reduces silica and titanium dioxide from slag. Therefore, further studies were carried out at 1500 °C. Subsequent experiments were conducted with a varying amount of wollastonite flux, i.e., 5, 10, 15, 20, 30, and 40 wt%, to optimize the amount of flux. The metal separation performance was decreased drastically with a decrease in the wollastonite addition below 20 wt%. However, clear slag-metal separation was observed in the samples with additions of wollastonite above 20 wt%. Therefore, 20 wt% of wollastonite was chosen as the optimized flux. This value is higher than the FactSage value, which may be due to the fact that a larger amount of flux or a higher temperature is required to make slag sufficiently fluid. Generally, it is better to conduct an experiment at a temperature sufficiently above the melting point.
The iron nugget formed during smelting was easily separable from the slag. Around 85 % of the iron, originally present in the bauxite residue, was extracted in the form of a nugget. Small iron particles that are still in the slag phase were subsequently separated after grinding by using a permanent magnet. Around 10 % of the iron could be extracted in this way. The chemical analysis of the impurities in metal nugget is given in Table 3 which shows that the nugget is rich in iron and can be used for steel making or cast iron production [18].
The chemical analysis of the slag sample is given in Tables 2 and 4. REEs analysis is shown in Table 2 for the sake of comparison. The concentration of REEs in the slag sample was increased by a factor of about 1.4 compared to the concentration of REEs in bauxite residue.
Slag Leaching Studies
Leaching experiments were conducted with different mineral acids (HCl, HNO3, and H2SO4) to evaluate the selectivity of the different elements. Initial leaching experiments were conducted at 25 °C with a liquid-to-solid (L/S) ratio of 50. A high L/S ratio was used to avoid filtration problems due to the formation of silica gel [10]. The acid concentration was varied from 0.25 to 6 N. The leaching experiments were conducted for a period of 24 h.
The dissolution of different elements during HCl leaching of the slag sample at different acid concentrations is shown in Fig. 9. It shows that with an increase in acid concentration, the extraction yield of REEs, except scandium, is increasing. The effect is prominent up to 1 N acid concentration and levels off at higher acid concentrations. Recoveries are lower at low acid concentration due to the high pH of the leach solution. The scandium extraction increases with increasing acid concentration up to 3 N, but then decreases at 6 N. This may be due to the absorption of scandium on silica, a phenomenon that was earlier described in the literature [26] and which is supported by the observed similarity in extraction behavior between Sc and Si. The maximum extraction yields are around 60 % for Y, La, Ce, Nd, and Dy, while it is around 40 % for Sc. The extraction of scandium is low compared to that of other REEs due to its different chemical behavior [6]. More than 80 % of Na, Fe, and Al are dissolved in the solution. Ca dissolution was around 80 %. Only around 20 % of Ti is soluble, even at 6 N. Si dissolution increases up to 0.5 N and then decreases from 3 N on, due to the precipitation of silicon hydroxides at high acid concentrations [27]. Similar results were observed for HNO3 leach solutions (Fig. 10). The scandium extraction yield was higher for HNO3 leaching (60 %) compared to HCl leaching. As with HCl leaching, a drastic decrease in the extraction of scandium was observed when leaching with 6 N of HNO3. The dissolution of iron also decreased with increasing acid concentration above 1 N, which is caused by the oxidation of Fe(II) ions by HNO3, which is an oxidizing agent. The extraction rates of the REEs were different for H2SO4 leach solutions compared with the other two acids (Fig. 11). The extraction rate of Y and Dy in sulfuric acid is similar to other acids but there is a decreasing extraction trend related with the increasing ionic radii, which may be due to the formation of a solid product layer (calcium sulfate, confirmed by SEM–EDX) in H2SO4.
Increasing the leaching temperature can increase the extractions due to enhanced reaction rates. Therefore, further leaching experiments were conducted at 90 °C. High-temperature leaching results are given in Fig. 12. The extraction of scandium now reached its maximum at 3 N of acid concentration. At 0.5 N, the extraction of scandium is very low, which is due to the high pH of the solution (~3). Extraction yields are high for Sc and Y (>90 %), followed by Dy and Nd (>80 %) and Ce and La (>70 %) at 3 N for both HCl and HNO3. Sulfuric acid leaching results are almost matching with the results reported in the literature [8, 10]. More than 70 % of Ti is extracted in the solution at 3 N for all the acids. Most of the Na, Al, and Fe are dissolving in the solution at 3 N, except for Ca, which barely (~20 %) dissolves in the sulfuric acid solution due to the low solubility of CaSO4. At the conditions of highest scandium extraction, the absolute amounts of scandium and iron concentration in the leach solution are 3 and 250 ppm, respectively.
HCl leaching of slag and that of bauxite residue are compared in Fig. 13. For ease of comparison, the dissolution of iron from the slag shown in the figure is expressed as the percentage of the amount of iron that was present in the original bauxite residue. The extraction results of REEs from slag are comparable with those from bauxite residue except for scandium at 0.5 N acid concentration. It is difficult to leach the REEs from the bauxite residue above 50–60 % without dissolving major part of the Fe. However, most of the REEs can be extracted from the slag with only 4 % of the Fe dissolution with respect to the amount present in the bauxite residue (i.e., almost complete Fe dissolution from the slag). Most of the Ca, Al, and Na and around 70 % of Ti are dissolved from the slag at 3 N acid concentration. Al and Ti can also be recovered from the leach solution together with REEs in order to make the process more sustainable.
Conclusions
Iron from Greek bauxite residue was successfully separated in the form of a metallic nugget at 1500 °C with 5 wt% graphite as reducing agent and 20 wt% wollastonite as flux. A graphite content above 5 wt% and a wollastonite content below 20 wt% decreased the slag-metal separation performance. Reduction of TiO2 affected the slag-metal separation. More than 95 % of the iron could be extracted from the bauxite residue. Room-temperature leaching of the slag sample gave low extraction yields, whereas high temperatures improved the extraction yields. All of the scandium, most of other REEs, and about 70 % of titanium could be leached at 90 °C using HCl and HNO3. Selectivity of scandium over other REEs is higher in the case of H2SO4 leaching. The main advantage of slag leaching compared to direct bauxite residue leaching is that most of the REEs can be extracted, with a minimum co-dissolution of iron, thus limiting cumbersome purification of the leachate.
References
Pontikes Y, Angelopoulos GN (2013) Bauxite residue in cement and cementitious applications: current status and a possible way forward. Resour Conserv Recycl 73:53–63. doi:10.1016/j.resconrec.2013.01.005
Binnemans K, Jones PT, Blanpain B, Van Gerven T, Yang Y, Walton A, Buchert M (2013) Recycling of rare earths: a critical review. J Clean Prod 51:1–22. doi:10.1016/j.jclepro.2012.12.037
Ochsenkuhn-Petropoulou MT, Hatzilyberis KS, Mendrinos LN, Salmas CE (2002) Pilot-plant investigation of the leaching process for the recovery of scandium from red mud. Ind Eng Chem Res 41:5794–5801. doi:10.1021/ie011047b
Mordberg L (1993) Patterns of distribution and behavior of trace elements in bauxites. Chem Geol 107:241–244. doi:10.1016/0009-2541(93)90183-J
Ochsenkühn-Petropulu MT, Lyberopulu T, Ochsenkühn KM, Parissakis G (1996) Recovery of lanthanides and yttrium from red mud by selective leaching. Anal Chim Acta 319:249–254. doi:10.1016/0003-2670(95)00486-6
Borra CR, Pontikes Y, Binnemans K, Van Gerven T (2015) Leaching of rare earths from bauxite residue (red mud). Miner Eng 76:20–27. doi:10.1016/j.mineng.2015.01.005
Fulford GD, Lever G, Sato T (1991) Extraction of rare earth elements from Bayer process red mud. US Patent 5030424
Logomerac VG (1979) Complex utilization of red mud by smelting and solvent extraction. Trav Com Int Etude Bauxites Alumine Alum 15:279–285
Logomerac VG (1979b) The complex utilization of red mud or low grade bauxite by solvent extraction. In: Proceedings of the international solvent extraction conference, pp 516-520
Sargic V, Logomerac V (1974) Leaching and extraction in the complex processing of red mud. Trav Com Int Etude Bauxites Alumine Alum 11:71–78
Guccione E (1971) Red mud, a solid waste, can now be converted to high-quality steel. Eng Min J 172:136–138
Misra B, Kirtepatrick D, Slavik M (2000) Pyrometallurgical extraction of alumina and iron from red mud. EPD Congress. Warrandale, TMS-AIME, pp 369–381
Liu P, Huo Z, Gua S, Ding J, Zhu J, Liu G (1995) Magnetic dressing iron mineral concentrate from Bayer red mud. Light Met 1995:149–153
Kumar S, Kumar R, Bandopadhyay A (2006) Innovative methodologies for the utilisation of wastes from metallurgical and allied industries. Resour Conserv Recycl 48:301–314. doi:10.1016/j.resconrec.2006.03.003
Paramguru RK, Rath PC, Misra VN (2005) Trends in red mud utilization – A review. Miner Process Extr Met Rev 26:1–29. doi:10.1080/08827500490477603
Panov A, Klimentenok G, Podgorodetskiy G, Gorbunov V (2012) Directions for large scale utilization of bauxite residue. Light Metals 2012:93–98
Mishra SK, Bagchi MN (2002) Mud to metal—Romelt is an answer.In: Smelting reduction for iron making. pp 167–170
Balomenos E, Gianopoulou I, Panias D, Paspaliaris I (2011) A novel red mud treatment process. Trav Com Int Etude Bauxites Alumine Alum 36:255–266
Process for the separation and recovery of Fe, Ti and Al values from ores and waste materials containing same. US Patent 2830892
Raspopov NA, Korneev VP, Averin VV, Lainer YA, Zinoveev DV, Dyubanov VG (2013) Reduction of iron oxides during the pyrometallurgical processing of red mud. Russ Metall 1:33–37. doi:10.1134/S0036029513010114
Erçag E, Apak R (1997) Furnace smelting and extractive metallurgy of red mud : recovery of TiO2, Al2O3 and pig Iron. J Chem Technol Biotechnol 70:241–246. doi:10.1002/(SICI)1097-4660(199711)70:3<241::AID-JCTB769>3.0.CO;2-X
Shaoquan X, Suqing L (1996) Review of the extractive metallurgy of scandium in China (1978-1991). Hydrometallurgy 42:337–343. doi:10.1016/0304-386X(95)00086-V
Bale CW, Bélisle E, Chartrand P, Decterov SA, Eriksson G, Hack K, Jung IH, Kang YB, Melançon J, Pelton AD, Robelin C, Petersen S (2009) FactSage thermochemical software and databases—recent developments. Calphad 33:295–311. doi:10.1016/j.calphad.2008.09.009
Borovský T, Kijac J, Buľko B, Domovec M, Havran J (2011) The effectiveness of dephosphorization with and without fluorspar using in the electric arc furnace steelmaking. Acta Metall Slovaca 17:263–268
Ding YG, Wang JS, Wang G, Ma S, Xue QG (2012) Comprehensive utilization of paigeite ore using iron nugget making process. J Iron Steel Res Int 19:9–13. doi:10.1016/S1006-706X(12)60119-8
Ma J, Wang Z, Shi Y, Li Q (2014) Synthesis and characterization of lysine-modified SBA-15 and its selective adsorption of scandium from a solution of rare earth elements. R Soc Chem Adv 4:41597–41604. doi:10.1039/C4RA07571D
Gorrepati EA, Wongthahan P, Raha S, Fogler HS (2010) Silica precipitation in acidic solutions: mechanism, pH effect, and salt effect. Langmuir 26:10467–10474. doi:10.1021/la904685x
Acknowledgments
This work was supported by a DBOF grant from KU Leuven to CRB and by the Research Platform for the Advanced Recycling and Reuse of Rare Earths (IOF-KP RARE3). YP is thankful to the Research Foundation Flanders (FWO) for the post-doctoral fellowship. The authors thank Aluminum of Greece for providing the bauxite residue sample.
Author information
Authors and Affiliations
Corresponding author
Additional information
The contributing editor for this article was B. Mishra.
Rights and permissions
About this article
Cite this article
Borra, C.R., Blanpain, B., Pontikes, Y. et al. Smelting of Bauxite Residue (Red Mud) in View of Iron and Selective Rare Earths Recovery. J. Sustain. Metall. 2, 28–37 (2016). https://doi.org/10.1007/s40831-015-0026-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-015-0026-4