Abstract
A new recycling process for the extraction of rare earths from neodymium–iron–boron (NdFeB) magnet scrap is being developed, based on the direct extraction of rare earths from end-of-life magnet material in a molten fluoride electrolysis bath. Rare earths are required in their metallic form for the production of new NdFeB magnets, and the suggested process achieves this through a single step. The process is being developed on a laboratory scale and has been proven to work in principle. It is expected to be environmentally beneficial when compared to longer processing routes. Conducting life cycle assessment at R&D stage can provide valuable information to help steer process development into an environmentally favorable direction. We conducted a life cycle assessment study to provide a quantitative estimate of the impacts associated with the process being developed and to compare the prospective impacts against those of the current state-of-the-art technology. The comparison of this recycling route with primary production shows that the recycling process has the potential for much lower process-specific impacts when compared against the current rare earth primary production route. The study also highlights that perfluorocarbon emissions, which occur during primary rare earth production, warrant further investigation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rare earth elements (REEs) play a key role for the advancement of green technologies, with permanent NdFeB magnets for efficient motors accounting for one of the most important applications in terms of REE market value [1]. Recent geopolitical REE supply risks have triggered an interest in their recycling, among other strategies followed to alleviate potential supply risks [2, 3]. While industrial NdFeB scrap recycling is already practiced, end-of-life scrap is generally shredded and the REE content lost into the steel fraction [1]. The diversities of magnet applications, component design, and material composition pose challenges to the development of collection, disassembly, and recycling processes, which would need to be established for the processing of end-of-life NdFeB magnets [4]. However, there is a strong demand for effective recycling of REE containing waste products, and REE recycling from NdFeB magnets is expected to play an increasingly important role in future REE supply [1, 5, 6]. Besides its role in mitigating REE supply risk shortages, recycling is expected to be beneficial over primary REE production from an environmental impact point of view for a number of processing options which have been analyzed in recent life cycle assessment (LCA) studies [7,8,9]. REEs are required in metallic form for NdFeB magnet production. The prominent process for REE metal production involves electrolysis of rare earth oxides (REOs), obtained through primary production routes [10]. Direct (magnet material recycling) or indirect recycling routes are being developed to recycle the magnet material as a whole, or to extract the REEs, respectively [2]. Direct recycling processes can be expected to be most environmentally advantageous due to the short processing routes, only surpassed by magnet reuse [6, 9]. For indirect metallurgical recycling, hydrometallurgical and pyro-metallurgical processes are distinguished. Indirect routes offer more flexibility in that they are less sensitive to contamination and variability of the input material stream. They can produce individual elements as outputs, but typically involve numerous processing steps in which the rare earths are separated from exogens (iron, boron, and impurities), and individually separated and reduced from rare earth oxides to metals [2, 11, 12].
A new recycling process has been developed, based on the direct extraction of rare earths from end-of-life (EOL) magnet material in a molten salt electrolysis bath, thereby eliminating the need to conduct a more complex, multistage process. This new process development is technologically challenging, but a single-step recovery of REEs from magnetic scrap is very attractive from an industrial point of view [13]. Various research groups are working on this topic [14,15,16,17,18]. Extraction processes are promising in that they are generally suitable to handle NdFeB scrap with variable compositions and contamination levels [19, 20]. Since the electrolyte is reusable and the processing chain short, the recycling process is also expected to be environmentally beneficial over longer processing routes [13, 20]. The process offers some convenient technical advantages over the current molten salt electrolysis process used in primary REE production: First, it solves the problem of low solubility of REO in molten fluorides by converting them to rare earth fluorides before subjecting them to the electrolysis. Second, the common problem of oxyfluoride formation from rare earth oxides (REOs) in molten fluorides, which decreases the efficiency of the electrolysis, is overcome. Third, the issue of fluorocarbon formation on the anode is resolved by employing a reactive anode instead of the conventionally used graphite anode, which is anodically dissolved to regenerate the fluorinating agent in situ in the electrochemical reactor.
Conducting a life cycle assessment study to support R&D can provide valuable information to help steer process development into an environmentally favorable direction [21,22,23,24]. To date, no LCA studies have been conducted for this new recycling process.
This paper presents an LCA study of a recycling process during which REEs are extracted from scrap magnets through molten salt electrolysis. The goal of this study was to help guide the development of this process, to identify the process-specific impacts associated with this route and to get an indication of how they compare against those of the most common primary production route for rare earths. The influences of important processing choices which affect the environmental impacts of the process being developed are discussed.
Process Description
The one-step recycling process to extract rare earth metals from scrap magnets is being developed on a laboratory scale at TU Delft. Nonmagnetized, uncoated magnet samples were used in the experiment. The magnets were ball milled under inert atmosphere to increase the surface area to facilitate the electrochemical reactions, and the powder was inserted into the salt bath. This key process step (i.e., the electrolysis) is the most technologically challenging one, and the focus of the research undertaken at TU Delft.
The experimental setup consists of an electrolytic cell with lithium fluoride used as an electrolyte. Aqueous solutions are unsuitable since the REE deposition potential is well below the water decomposition potential, and the major reaction would thus be the decomposition of the electrolyte rather than REE deposition [13]. The cell is positioned within a resistance furnace used to heat the bath to obtain molten lithium fluorides (LiF). An iron anode and a molybdenum cathode are used. The milled magnet powder from which the REEs are to be extracted is added to the electrolyte. The process temperature (~ 950 °C) is maintained throughout the process, which is conducted under a protective argon atmosphere to prevent oxidation of the extracted REEs.
Neodymium fluoride (NdF3) is added to the mixture as catalyst to initiate the electrolysis process. Current (from a different circuit to the one heating the resistance furnace) is applied to start the electrochemical reactions, which first decomposes the NdF3 (Eq. 1) since it is less stable than LiF. Nd3+ is reduced on the cathode, and F2 ions are oxidized at the anode; hence, FeF3 is formed on the anode (Eqs. 2 and 3). The anode is consumed in this process.
FeF3 then reacts with the REE contained in the molten magnet material, and the REE in the magnet material is exchanged for Fe from the FeF3 to form REF3, thereby extracting and separating the rare earths from the iron and boron (and other minor constituents such as Al, Co, etc.) (Eq. 4). The fluoride ions are thereby liberated and then move on to the anode, which maintains the reaction. The additional Fe in the magnet material takes the form of an intermetallic phase, such as Fe2B or FeCo [25].
The cell voltage causes the REF3 to dissociate again (Eq. 1), after which the rare earths are reduced at the cathode and are deposited in layers of different REEs, with the (electrochemically) favored reaction happening first (Eq. 2).
A variation of the process uses the scrap magnet directly, i.e., the magnet material is not milled prior to the electrolysis, but inserted directly into the electrolysis bath in lieu of the iron anode. Hence, the scrap magnet is simultaneously oxidized (Eq. 4) and anodically dissolved, and reacts with FeF3, which is added directly into the bath as fluorinating agent. As a result of this reaction, NdF3 is formed, which will subsequently dissociate again (Eq. 1).
An unintended side reaction may occur in both process variants, if the FeF3 is decomposed before it reacts with the scrap magnet material. In this case, the Fe would be deposited at the cathode, and the fluoride ions would maintain the anodic reaction. To what extent this happens requires further research. However, REE-Fe alloys can be directly used in magnet production.
Experimental SetUp
For the experiment, lithium fluoride (98.5%; Alfa Aesar) was mixed in a glove box with the NdFeB magnets (supplied by Magneti Ljubljana), previously ball milled into fine powder. The magnet composition as provided by Magneti Ljubljana is shown in Table 1. 15 g of milled magnet powder were processed in one experiment. Neodymium fluoride (99.9%; Alfa Aesar) was added in order to initiate the electrolysis process. This mixture was then charged into a graphite crucibleFootnote 1 and heated up to 950 °C for 3 h under argon atmosphere, with an applied current of 20 A for the resistance furnace, and 15 A for the processing current driving the electrolysis [10].
After completion of the electrolysis, the samples were analyzed by X-ray diffraction (XRD) as well as by Electron Probe Micro Analysis (EPMA) in order to determine the phases that are formed during the experiments and their compositions. The conversion of the neodymium from the magnet into neodymium fluoride was earlier proved in the same lab [26]. The results from XRD and EPMA show the deposition of neodymium and dysprosium on the molybdenum cathode.
The experiments showed that the reaction between the magnet material and FeF3 (Eq. 4) works with a very good efficiency in a lab setting. Furthermore, it could be shown that neodymium and dysprosium can be extracted from the magnet material, i.e., a REE mischmetal product can be produced at the cathode, which corresponds to the composition of the REEs in the magnet. The REMs (rare earth metals) are deposited individually in layers.
The process development is challenging due to the high reactivity of rare earths at high temperatures: Despite the protective atmosphere, the REEs are currently oxidized at the cathode. So far, it could be shown that the process is feasible in principle. Further experiments are required to determine the recovery yield in lab- and pilot-scale environments. Furthermore, the degree to which unintended side reactions occur remains to be tested.
Methods: Life Cycle Assessment
A life cycle assessment study was conducted in accordance with the goal and scope definition (Goal of the Study, Considered Scope and System Boundaries section). For the life cycle inventory (LCI) compilation, qualitative information on the process was obtained in close collaboration with the researcher developing the process. Since at the time of writing, the process development so far had focused mainly on the proof of principle, but had not yet been implemented on an industrial scale, assumptions were required to compile the foreground data (i.e., the process-specific material and energy consumption, waste, and emissions). They were based on relevant literature regarding primary rare earth production and aluminum production, and discussions with experts in the group developing the process. To account for uncertainties associated with the process-specific impacts of the foreground process, a range of values are presented in the LCI.
The modeling was done in openLCA (Version 1.4.2). Ecoinvent V3.2 (APOS) data were used to model the background processes.Footnote 2 A standard set of impact assessment methods (CML baseline, Version 4.4. of January 2015) was used for the assessment.
Goal of the Study, Considered Scope, and System Boundaries
The aim of this study was to conduct a (gate-to-gate) life cycle assessment study in order to determine the potential impact of the one-step recycling process of REEs from scrap NdFeB magnets through molten salt electrolysis. Process developers can be informed about the potential impacts of the process being developed, and about the aspects that should be given special attention from the point of view of reducing overall process impacts. Since the process is at an early stage of development, the analysis cannot present an exact figure. Rather, the influence of different factors can be tested. The environmental impacts from this process route can also be tentatively compared against those of alternative primary production processes for REEs.
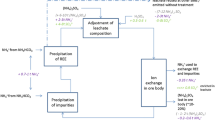
Nd and Pr make up the bulk of the rare earth fraction in Nd–Fe–B magnets. Since the focus of this study was on the analysis of process-specific impacts, the functional unit of this analysis was chosen as the production of one kg Nd–Pr alloy through extraction from NdFeB magnet scrap. From a technical point of view, the process is similar for all REEs. The technical system includes the one-step molten salt electrolysis recycling process, starting with the extraction of the magnet from its compound (e.g., a motor), demagnetizing, decoating and ball milling (Fig. 1). The product is an REM alloy, at purity levels sufficient for use in magnet production. The first life of the magnets, collection, and transport of end-of-life magnet applications to the recycler are not included in the analysis.Footnote 3
Processing steps for the EOL-magnet recycling process—two process variants, with and without milling, and for the primary production process which was used for a rough comparison (Bayan Obo) [8]
The extraction of the magnets from EOL compounds, demagnetizing and cleaning steps were not conducted in the lab, but would be required if the process was implemented in practice. They are therefore included in the LCI. The focus of this study is on the electrolysis process since it is the most technologically challenging processing step, and the focus of research efforts of the researcher developing the process. Data are compiled from the literature and complemented with expert discussions held with the researchers developing the process, and data provided by a magnet manufacturer.
Life Cycle Inventory Recycling Process: Assumptions
Expected (Qualitative) Differences Between Lab- and Industrial-Scale Setups
When using EOL magnets as input material for the electrolysis, the magnets need to be extracted from their compounds (e.g., motors), demagnetized, cleaned, and milled.
The process being developed closely resembles the Chinese state-of-the-art molten fluoride electrolysis process for rare earth reduction [27]. Contrary to the laboratory setup, which uses a resistance furnace to obtain the required temperature of the salt bath and a power source to drive the electrolysis, the heating and the current would both be supplied from the same power source in an industrial setup (Yang Y, 2016, personal communication). The current is fixed and maintained at a certain level. Current efficiency, defined as the percentage of the cell current utilized to deposit the target metals at the cathode, effectively varies over time. Among other factors, this depends on the extent to which side- or back-reactions happen, impurities are deposited, and short circuits occur [28]. Since the same current drives both the heating and the electrolysis process, some slight temperature variation is to be expected. The cell resistance may increase during the operation, e.g., due to an increase in impurities in the cell [29].
In previously conducted lab-scale experiments, the focus has been on proving process feasibility, rather than optimizing energy and material utilization. For this reason, the electrolyte is exchanged for each experiment conducted. If the process was implemented on an industrial scale, the electrolyte would be reused [13]. The infrastructure, including the electrodes, electrolyte and the bricks which are part of the cell construct, would typically be replaced every 5 months [10].
It was assumed that the typical Chinese 3 kA technology is representative of the technology size of the equipment which would be used for the NdFeB recycling when implemented on an industrial scale.Footnote 4 It has been mentioned in the literature that the process temperature is a factor considered in optimization efforts for the industry process [30]. The process developed at TU Delft can be conducted at a slightly lower temperature than the industrial-scale reduction of REO to REM. Since the differences in temperature are expected to be small, the industrial setup is assumed to be a representative estimate.
Extraction of the Magnet from the Compound, Demagnetizing, and Decoating
The life cycle inventory data for the extraction of the magnet from the compound, demagnetizing, and decoating are based on a previously conducted life cycle assessment study. The magnets are separated from the permanent magnet motors with the help of pressurized air; demagnetized with infrared light, and cleaned/polished with silicon carbide and reusable polyphenylene sulfid granulate. [9] A detailed description of the processing steps analyzed in this study can be found in [9, 11]. The data (presented in SI Table 2), based on the extraction of magnets from traction motors used in electric cars, are used here as a proxy for different end-of-life NdFeB applications. It should be noted that the losses of magnet material during the extraction from its compound could, in practice, differ between EOL-NdFeB applications. The effective removal of the magnets will, in practice, depend on the development of suitable dismantling and/or extraction processes. Successful trials have been undertaken for some applications, which, besides traction motors, include hard disk drives and air compressor motors [31, 32].
Ball Milling of Magnet Material (Process Variant 1 Only)
After cleaning, the material is milled under protective argon atmosphere to prevent oxidation. The milling step is undertaken to increase the surface area to facilitate the rare earth extraction. The energy consumption of the ball-milling step can be estimated from the hardness of the material being milled, and the particle sizes before and after the milling [33, 34] (SI Table 2). The grain size produced in the lab experiment is ~ 200 µm.
Electrolysis
Magnet Composition
The magnets can vary with regards to their content of individual rare earths. Usually, Nd, Pr, Dy, and Tb, and sometimes Gd are present in NdFeB magnets. REEs have very similar electrochemical properties, and therefore show similar behavior in the process. No distinction is made for magnets with different REE compositions for the purpose of this analysis, only the recovery of Nd and Pr was modeled for the purpose of this study.
Process Yield
With the current state of the lab experiments, detailed information on process yields for the reduction step, i.e., the fraction of the rare earth material contained in the magnet material which can be harvested as rare earth metal deposited at the cathode, is not yet available since the focus of the research has so far been on the proof of principle. Hence, it is also not yet known to what extent the milling of the magnet material improves the yield. Therefore, a wide range from 50 to 95% was modeled. The 95% corresponds to the best yield reported in the literature for primary REM production [27]; the 50% represent a conservative estimate.
Energy Consumption
In rare earth primary production, reducing RE oxides to metal is the processing stage which consumes most process energy per kg REM [35], and research is being undertaken to improve the current efficiency for rare earth electrowinning processes with alkali halides [36]. Energy is required to both drive the extraction via a direct current and maintain the temperature at which the reaction takes place (around 950 °C). The energy consumption was assumed to be continuous throughout the lifetime of the electrolytic cell (150 days).
The theoretical electricity consumption required for the deposition current can be calculated using Faraday’s law [28, 35], according to which for each type of metal, the (theoretical) amount which is deposited can be calculated for a given plating time and amperage. In practice, losses occur due to side reactions etc. A current efficiency factor of 65–78% is representative of the industrial fluoride system [37, 38]. The power consumption for the DC current driving the reaction is around 9–13 kWh/kg REM for industrial processes, with a material yield of 90–95% [10, 27, 35]. According to [35], the energy requirement is on the higher end of this range for mischmetal and lower for individual REM.
During plating time, the temperature has to be maintained—hence, the required plating time, which is a function of the amount of material processed, is approximately proportional to the energy requirement for heating (if the initial warm-up is neglected). Unlike in the lab experiment, in industrial electrolysis processes, the heat is provided by the same power source as the decomposition current. For the Chinese “3 kA technology,” the current state-of-the-art rare earth reduction technology, around three quarters of the total energy is used for obtaining and maintaining the temperature, while only 26% is attributable to the decomposition current [10, 27, 39]. Heat is lost through various routes; a detailed heat balance calculation is presented in [10]. The energy consumption range reported in the literature was adopted in this study. The value given in the literature refers to the REE output and is reported (in kWh/kg REM produced). It was assumed that in the case of a low recovery rate (50% assumed) the processing of the magnet would take the same amount of time as in the case of a higher (95% assumed) recovery rate, with approximately half the yield. Due to the fact that most of the electricity is attributable to heat loss, which is related to the process duration, and since it is not clear what additional side reactions would occur, it is assumed that the energy values given in the literature correspond to the rare earth content in the magnet (i.e., the input material), rather than the fraction recovered at the cathode. This means that the electricity consumption per unit of REM alloy during the electrolysis roughly doubles for the lower recovery rate.
Electrolysis Material Consumption and Disposal, Including Auxiliaries and Infrastructure
According to Vogel et al. [27], citing Zhang et al. [40], the 3 kA cell infrastructure is typically replaced every 5 months. In this period, around 10 t (8.5–11.5 t) of REM are produced. It was assumed that the complete infrastructure, including electrolytes and outer structure, is replaced.
Literature data were used as a basis for an estimate of the net electrolyte consumption. An estimate in the literature states that the net usage of LiF corresponds to approximately 1–1.5% of the REM output weight [10]. This is in line with the figures reported for similar processes elsewhere in the literature: Sprecher [8] published a dataset for primary rare earth production, where some of the assumptions were based on aluminum reduction process data, which they adjusted, based on differences in molecular weights. According to their dataset, 0.001 kg cryolite is used per kg of liquid Nd in the electrolysis process, and 0.01 kg of aluminum fluoride (with cryolite and aluminum fluoride used as proxy substances for the salts used in REM production). The total net salt consumption amounts to 1.3 wt% of the REM content processed. In aluminum production, the cryolite consumption is around 0.05 and 1.75 wt% of the output weight of the recovered aluminum, respectively [41, 42]. The estimate for this study was based on the data from Siming et al. [10], assuming that the LiF consumption is proportional to the REM content of the input material processed (SI Table 3).
Emissions
Contrary to primary REE production, the recycling process causes no carbon monoxide or carbon dioxide emissions during the anodic reaction, since instead of the graphite anode, an iron anode is used (Eq. 3). Perfluorocarbon (PFC) emissions are an issue in aluminum production, and there is indication that they might present an issue in primary rare earth production, too—see Perfluorocarbon (PFC) Emissions. In the recycling process, no carbon/graphite anodes are employed. The crucible is typically made from graphite, thereby introducing carbon into the cell. Alternative materials which could withstand the corrosive nature are more expensive. However, the pathways which produce PFC emissions in aluminum reduction cells all refer to the anode(s) per se [43]. Therefore, it was assumed that no PFC emissions are generated from the recycling process.
Assumptions Metal Scrap Recycling
Regarding the EOL magnet, no burdens from the first life of the magnet (original production of the magnet) are assigned to the scrap magnet entering the system. The iron which is left over after the electrolysis from the magnet scrap is assumed to be further processed.
Life Cycle Inventory: Primary Production—Data Assumptions
To obtain a tentative comparison, the production of Nd and Pr from the recycling process are compared against the primary production of Nd and Pr from Bayan Obo. Life cycle inventory datasets for primary rare earth metal production have previously been compiled [8, 44,45,46,47]. Process-relevant parameters (energy and/or material consumption) are also reported outside the life cycle assessment literature [27, 35]. The last step in the rare earth metal production process, i.e., the electrolysis, is associated with high-energy requirements, most of which are accountable to heat losses [10].
In this study, the primary production of rare earth oxides is based on the data from Sprecher et al.’s baseline scenario [8]. The data are provided in the original publication. However, the baseline dataset was adjusted as indicated in this section, and in SI Table 1.
Update of Electricity Consumption Figures
To account for the range in electricity consumption values reported in the literature, the figures in the primary production dataset were adjusted in order to align them with the assumptions made for the recycling process.
Update of Allocation Factors
The allocation of environmental flows to Nd and Pr was updated. Value allocation was used to share the process impacts from both multi-output stages, reflecting prices from recent years (2013–2016). There are two stages in the primary production of rare earths for which assumptions regarding coproductions are necessary: the beneficiation stage, where concentrated iron ore and rare earth concentrate are separated, and the solvent extraction stage undertaken to separate individual rare earths. For the beneficiation stage, around 74% of the output value is attributable to the mixed rare earth concentrate [48], and around 77% of the rare earth value after separation is attributable to the “magnet REEs,” i.e., Nd and Pr (see SI Table 4 for data used to calculate the updated allocation factors for the allocation between individual REEs).
Perfluorocarbon (PFC) Emissions
Perfluorocarbon emissions are potent and very long-lived greenhouse gases associated with aluminum and semiconductor production [49]. During aluminum electrolysis, PFCs are formed in unintended side reactions when the fluorine from the salt bath reacts with the carbon from the anode. Similar effects also occur during rare earth reduction, where graphite anodes are also state of the art [29]. The effects are, however, far less discussed compared to the PFC emissions from aluminum and semiconductor production, since the global production volumes for rare earth metals are small ([43, 50]).
PFC emissions cannot be captured once released, so the formation has to be managed via the control of process conditions [39]. Both in aluminum and rare earth production, the formation processes are complex and influenced by a multitude of factors [39, 43]. A patent has been filed to address this issue back in 1998 [51, 52]; but the solution was later claimed impracticable [53]. Elsewhere in the literature, PFC emissions associated with rare earth production have also been acknowledged as an issue which merits further investigation [54, 55]. Inert anodes (which would eliminate the carbon anode), combined with new types of electrolytes, are a research topic in molten fluoride electrolysis [56, 57].
PFC emissions given in published life cycle inventories for rare earth electrolysis are considered to be of similar magnitude to those of aluminum production [8, 45], or not listed in the inventory [44].
In recent years, a noticeable gap between reported and measured PFC emissions has emerged, and efforts are being made to improve emissions accounting for PFCs from aluminum production [43, 49, 58]. Recent work by Vogel et al. [39, 59] provides estimates for PFC emissions from rare earth production based on a technology review and lab studies. Their findings suggest a possibility for high PFC emissions from rare earth production (when compared against emission factors for aluminum in CO2-equ. per kg of metal produced). The authors recommend the implementation of industrial measurements of PFC emissions in rare earth smelters [59]. Two publications present measurements of PFCs in industrial settings in Chinese REM electrolysis plants [60, 61]. For those plants, GWPs from PFC emissions (in CO2-equ. per kg of REM metal) were found to be of similar magnitude to those of aluminum metal.
For this study, the recent industrial measurements for PFC emissions were adopted for the optimistic estimate. For the pessimistic estimate, the emission factors from the medium emission scenario in [59] were adopted to consider possibly higher emissions that might occur in an illegal or unregulated smelting plant.
Hydrogen Fluoride (HF) Emissions
Hydrogen fluoride is produced during aluminum production and during rare earth electrolysis [10, 62]. When contacted with water vapor, hydrogen fluoride forms hydrofluoric acid. Both the gas and acid are toxic to humans, marine, and freshwater species. Fluoride emissions can be managed via process control and through the use of fume control systems [62]. In aluminum production, HF emissions can be efficiently scrubbed with a very high efficiency of ca. 99% [63]. Details on the management of hydrogen fluoride (HF) emissions from rare earth electrolysis could not be found in the literature; however, they are mentioned in the literature as an issue [57]. According to Vogel et al. [39] and Schreiber et al. [45], in rare earth production, the emissions are managed via scrubbing. Siming et al. [10] reports that HF emissions in rare earth electrolysis are being addressed by the development of new cell designs. The figure provided in [8] was adopted for this study.
Results
The life cycle impact assessment was conducted with CML baseline. Results are shown for a selection of impact categories, namely ozone depletion, human toxicity, depletion of abiotic resources (elements, ultimate reserves), acidification, depletion of abiotic resources (fossil fuels), photochemical oxidation, climate change, and eutrophication. The results from the ecotoxicity categories are not shown in the results table (Table 2), which is justified in the next section.
Comparing the Proposed Recycling Process Against Primary Rare Earth Production
The results of the recycling route were compared against the most common primary production route for Nd and Pr. Due to the nature of the study, this can only be considered a rough comparison.
Results indicate that recycling is beneficial for the analyzed impact categories and almost all scenarios, with the exception of the most pessimistic recycling scenario (Table 2). When compared against primary production, the results for this most pessimistic recycling scenario with only 50% REM recovery and milling indicate that the impacts would be lower than those for the primary production route for the majority of impact categories—with the exception of the result for the eutrophication category. In this recycling scenario, eutrophication is mainly due to electricity production, with more than half of the impact originating from the preparation of the scrap magnet material before the electrolysis.
Furthermore, results for the impact categories terrestrial, freshwater, and marine ecotoxicity did not confirm this trend for all scenarios. However, the contribution analyses conducted for these categories raised some doubts about the meaning of these results. For example, for the category terrestrial ecotoxicity, the production of REM from the recycling route was associated with higher impacts than primary production when the high (pessimistic) LCI estimate recycling scenarios with milling. Results show that for the recycling scenarios with milling, this category is dominated by the steel used for the iron anode. This steel is not required for the scenarios where the scrap magnet is directly used as anode. The impact is largely from chromium emissions to air during steel production, although a low-alloyed steel dataset was used here (as a proxy for silver steel). This result is very questionable for two reasons: First, the steel consumption is based on a conservative assumption, i.e., a very generous amount of steel was assumed, especially for the high (pessimistic) LCI estimate (see SI Table 3). Second, and most importantly, the addition of chromium to the steel is not crucial for this application.
Besides the findings from the contribution analysis, it should be noted that the ecotoxicity categories (terrestrial, freshwater, and marine ecotoxicity) are reportedly associated with high uncertainties [64], and therefore often omitted by LCA practitioners. The marine ecotoxicity in particular is not recommended for use for this reason.
Contribution Analysis GWP 100a: Recycling Process
Metal production is an important contributor to global warming [65]. It is therefore important to understand to what extent recycling processes provide an advantage regarding this issue over primary production [26, 66]. For this study, the focus is on identifying the environmentally important parameters in process development.
Against this background, a contribution analysis for GWP 100a is presented. Results refer to the production of 1 kg Nd–Pr (alloy). For the recycling processes, a contribution analysis for the best- and the worst-case scenario is presented.
The best-case recycling scenario is the low (optimistic) LCI estimate with 95% material recovery during electrolysis and without milling. 57% are attributable to the electrolysis, with 54% attributable to electricity for electrolysis and 3% for material and infrastructure (Nd oxide, LiF, etc.). A surprisingly large contribution is from the preprocessing (43%, of which 22% is attributable to demagnetization, and 18% to silicon carbide used for cleaning). For the worst case recycling (high (pessimistic) LCI estimate, 50% REE recovery, with magnet milling), only 40% are attributable to the electrolysis (33% electricity for electrolysis), and the other 60% to cleaning with silicon carbide and demagnetization.
Contribution Analysis GWP 100a: Primary Production
For the low (optimistic) LCI estimate of the primary production process, around 16% of the impact (GWP 100a) is from the electrolysis step, of which 0.4% is from direct emissions of PFCs, and 0.6% from upstream process emissions of this processing step (infrastructure, salt etc.). The other 14% are attributable to electricity consumed for the electrolysis process step. Around 84% are attributable to the processing steps before the electrolysis (mining, beneficiation, roasting, leaching, and solvent extraction). For the high (pessimistic) LCI estimate of the primary production process, 76% of the GWP 100a impact is attributable to direct PFC emissions from the electrolysis process, with only 5% attributable to the electricity consumption in the electrolysis process, and 19% attributable to the production of the rare earth oxides.
Sensitivity of LCIA Results to Different Factors
Primary Rare Earth Production
For the primary rare earth production, an optimistic and a pessimistic variant of the LCI dataset were modeled. A single baseline dataset was used as a reference for the primary production. The differences between the optimistic (low) and pessimistic (high) LCI estimates originate from the changes in this baseline dataset, namely the electricity consumption during the electrolysis and the PFC emissions. The PFC emissions only impact the results in category GWP 100a, but for this category, the results are extremely sensitive to the PFC emission estimates, with a factor 4 between the optimistic (low) and pessimistic (high) LCI estimates. This illustrates the strong influence of the conservative PFC emission factor, representing unregulated/illegal production. For other impact categories, the electricity consumption is mainly responsible for the differences, which amount to between 0 and 63% between the low and high estimates across the impact categories.
To check the sensitivity of the LCIA results to price fluctuations, prices from different time periods (and for different places in the supply chain) were used, but the results were not sensitive to this choice. Both a 1-year average FOB price for 2014/2015 (the price which reflects cost of production and transport to the harbor, but excludes shipping) and a 3-year average price in US$ for 2013/2016 were used (see SI Table 4 for details). However, the difference was small, with 77.2 and 76.6% of the rare earth values attributable to Nd and Pr, respectively.
Recycling
For the recycling process, an optimistic and a pessimistic variant were modeled as for primary production. In addition, the scenarios were varied with regards to the inclusion of a milling step, and regarding the recovery rates (see SI Table 2 and SI Table 3 for the LCI datasets).
When comparing the pessimistic (high) LCI estimates against the optimistic (low) estimates for the recycling process, the impact assessment results increase by 70–92% on average across all impact categories (Table 2). This is due to the LCI dataset entailing a large variation between optimistic (low) and pessimistic (high) LCI values for the pretreatment steps. These differences show up in the results, especially for the scenario with milling and only 50% recovery, which enhances these differences. Within the pretreatment steps, factors which noticeably impact the results include the energy consumption for demagnetization, the assumed argon consumption, material losses assumed for the milling process, and the quantity of silicon carbide used for magnet cleaning.
The influence of the inclusion of a milling step on the environmental impacts of the recycling processes is 25% on average for most impact categories (not considering human toxicity and ADP elements). These two impact categories are particularly sensitive to the inclusion of the milling step, and the difference in impact is therefore larger, which can be explained by some of the components in the steel used as anode material in the milling scenarios.
The results are, unsurprisingly, sensitive to the recovery rate. As an optimistic estimate, it was assumed that 95% of the rare earth elements can be recovered during the electrolysis step. If the recovery rate was substantially lower (50% was assumed), the impacts per kg Nd–Pr alloy increase approximately by 85% on average which varies depending on the impact category and scenario considered.
Summary and Conclusion
A life cycle assessment study was conducted for a one-step molten salt electrolysis process employed for the recycling of rare earths from scrap magnet material. The study was conducted to identify potential environmental hotspots early on during the process development, and to provide a rough indication of how the potential impacts of this secondary rare earth production route would compare to the impacts of the primary production routes. The results from this paper should be interpreted as an early estimate of potential impacts associated with the process being developed that can serve as a basis for further investigations.
The comparison of this recycling route to primary production shows that the recycling process has the potential for much lower process-specific impacts compared to the current rare earth primary production route. Since the recycling process is at an early development stage, the main focus has been on demonstrating that the process works in principle. Therefore, the influences of different processing choices and data assumptions on the results were tested. Even when a low REE recovery rate of 50% during the electrolysis is assumed, the recycling process is environmentally friendly for the majority of impact categories. Results show that the material recovery rate is crucial to the overall impact of the recycling process. Furthermore, the choice of preparation steps also influences the overall impact. In contrast to this, the cell infrastructure, which has been included in the study due to a relatively frequent need for replacement (every 150 days), does not have a big impact on the overall results. For the preparation steps, data assumptions should be further redefined in future studies. For the cleaning step in particular, which does not appear to be negligible in terms of impact contribution, it is recommended to further investigate what level of cleaning is actually necessary before the electrolysis.
The inclusion of the milling step can play a crucial role, but the difference is particularly large for the toxicity categories, and due to the assumed composition of the iron anode rather than the milling itself. The ecotoxicity categories are characterized by high uncertainties, and the additives in the steel which drive the impacts are not necessarily required in the anode material. Furthermore, it must be highlighted here that the purpose of the inclusion of the milling step is to increase the material recovery rate of the process. To what degree this happens could not yet be considered in these scenarios, since this had not been analyzed by the researchers developing the process until the time of writing; i.e., the milling step and the material recovery rate were modeled as if they were independent. However, this is an important knowledge gap without addressing which it is difficult to draw conclusions about whether the magnet should be milled or not from an environmental impact point of view. Thus, process development should focus on optimizing material recovery, and investigate to what extent milling improves the recovery rates.
Literature focusing on technical process improvement in rare earth primary production mentions potentially large additional impacts from PFC emissions in primary rare earth production which have not yet been discussed in LCA literature. PFC emissions from the rare earth industry are currently not reported, but have very high global warming potentials, i.e., even small quantities emitted could make a large contribution to the overall impact. Recent measurements in rare earth smelting plants found PFC emissions to be of similar magnitude to those in aluminum production, i.e., on the lower end of the range assumed for this study. However, due to a potential for high emissions in the absence of process control, further PFC emission measurements are recommended. If this is shown to be an issue, rare earth producers should be encouraged to report PFC emissions, as is done by aluminum manufacturers [43].
Notes
Fluoride is very corrosive, thereby limiting the options for materials which can be used for the crucible. On an industrial scale, graphite crucibles are commonly used. For research purposes, inert metals such as Mo, Ni, Pt, and W can be used.
Background processes are processes not directly related to the recycling process itself, such as the production of chemicals and electricity used in the process.
The cutoff allocation method was used here, i.e., the impacts from the first life cycle of the magnet are not accounted for, and the material enters the recycling process burden free. The approach was chosen since the focus of this study was on technology development and thus impacts associated directly with the process being developed.
Although larger cells have been developed, the data given in the literature are for the 3 kA cells which are said to be the most common technology as of now.
References
Yang Y, Walton A, Sheridan R et al (2016) REE recovery from end-of-life NdFeB permanent magnet scrap: a critical review. J Sustain Metall. https://doi.org/10.1007/s40831-016-0090-4
Binnemans K, Jones PT, Blanpain B et al (2013) Recycling of rare earths: a critical review. J Clean Prod 51:1–22. https://doi.org/10.1016/j.jclepro.2012.12.037
ERECON (2015) Strenghtening the European rare earths supply chain: challenges and policy options: Kooroshy J, Tiess G, Tukker A, Walton A (eds). ERECON, Brussels
Elwert T (2015) Entwicklung eines hydrometallurgischen Recyclingverfahrens für NdFeB-Magnete. Dissertation TU Clausthal. https://doi.org/10.21268/20150513-093644
Schulze R, Buchert M (2016) Estimates of global REE recycling potentials from NdFeB magnet material. Resour Conserv Recycl 113:12–27
Nlebedim IC, King AH (2018) Addressing criticality in rare earth elements via permanent magnets recycling. JOM 70:115–123
Jin H, Afiuny P, McIntyre T et al (2016) Comparative life cycle assessment of NdFeB magnets: virgin production versus magnet-to-magnet recycling. Proc CIRP 48:45–50
Sprecher B, Xiao Y, Walton A et al (2014) Life cycle inventory of the production of rare earths and the subsequent production of NdFeB rare earth permanent magnets. Environ Sci Technol 48:3951–3958
Walachowicz F, March A, Fiedler S et al (2014) Verbundprojekt: recycling von elektromotoren—MORE: Teilprojekt: Ökobilanz der Recyclingverfahren. Projekt gefördert im Rahmen des Programms “Schlüsseltechnologien für die Elektromobilität (STROM) des BMBF
Siming P, Shihong Y, Zongan L et al (2011) Development on molten salt electrolytic methods and technology for preparing rare earth metals and alloys in China. Chin J Rare Met 3(35):3440–3450
Bast U, Blank R, Buchert M et al (2015) Recycling von Komponenten und strategischen Metallen aus elektrischen Fahrantrieben: Kennwort: MORE (motor recycling)
Önal MAR, Borra CR, Guo M et al (2015) Recycling of NdFeB magnets using sulfation, selective roasting, and water leaching. J Sust Metall 1:199–215
Abbasalizadeh A et al (2016) Rare earth extraction from NdFeB magnets and rare earth oxides using aluminum chloride/fluoride molten salts. In: Borges de Lima I, Filho WL (eds) Rare earths industry technological, economic, and environmental implications. Elsevier Inc, Amsterdam
Abbasalizadeh A, Teng L, Seetharaman S (2015) Neodymium extraction using salt extraction process. Miner Process Extr Metall 124:191–198
EREAN (2015) European rare earth magnet recycling network: EU FP7 marie curie initial training network. http://www.erean.eu/project.php
Hua Z, Wang J, Wang L et al (2014) Selective extraction of rare earth elements from NdFeB scrap by molten chlorides. ACS Sustain Chem Eng 2:2536–2543
Okabe TH, Sakaye S (2012) Method and apparatus for recovery of rare earth element. US patent 8,323,592 B2
Ryu H-Y, Lee Jong-Hyeon, Kim Wan-Gou, Nersisyan HH et al (2015) Electrochemical behaviour of Nd in its pyrometallurgical recovery from waste magnet. Rare Met 34(2):111–117
Firdaus M (2016) Review of high-temperature recovery of rare earth (Nd/Dy) from magnet waste. J Sust Metall 2(4):276–295
Takeda O, Nakano K, Sato Y (2014) Recycling of rare earth magnet waste by removing rare earth oxide with molten fluoride. Mater Trans 55(2):334–341
Miller SA, Keoleian GA (2015) Framework for analyzing transformative technologies in life cycle assessment. Environ Sci Technol 49(5):3067–3075. https://doi.org/10.1021/es505217a
Schrijvers DL, Leroux F, Verney V et al (2014) Ex-ante life cycle assessment of polymer nanocomposites using organo-modified layered double hydroxides for potential application in agricultural films. Green Chem 16(12):4969–4984. https://doi.org/10.1039/c4gc00830h
Villares M, Işıldar A, van der Giesen C et al (2017) Does ex ante application enhance the usefulness of LCA?: A case study on an emerging technology for metal recovery from e-waste. Int J Life Cycle Assess 22(10):1618–1633. https://doi.org/10.1007/s11367-017-1270-6
Wender BA, Seager TP (2011) Towards prospective life cycle assessment: Single wall carbon nanotubes for lithium-ion batteries. In: ISSST Chicago. https://doi.org/10.1109/issst.2011.5936889
Abbasalizadeh A, Malfliet A, Seetharaman S et al (2017) Electrochemical extraction of rare earth metals in molten fluorides: conversion of rare earth oxides into rare earth fluorides using fluoride additives. J Sust Metall 6:627–637. https://doi.org/10.1007/s40831-017-0120-x
Abbasalizadeh A, Seetharaman S, Sietsma J et al (2017) Electrochemical recovery of rare earth elements from magnets: conversion of rare earth based metals into rare earth fluorides in molten salts. Mater. Trans. 48(7):400–405
Vogel H, Friedrich B (2015) Development and research trends of the neodymium electrolysis—a literature review. In: Proceedings of EMC 2015. Development and research trends of the neodymium electrolysis
Yang Y (2012) Electrometallurgy—electrowinning, electrorefining, and molten salt electrolysis: metals production, refining and recycling (MPRR). Department of Materials Science and Engineering, WB3310TA: Extractive Metallurgy
Lucas J, Lucas P, Le Mercier T et al (2015) Rare earths—science, technology, production and use, 1st edn. Elsevier, Amsterdam
Zhang XL, Liu HX, Zheng X et al (2013) Influence of temperature distribution on products quality in 3KA rare earth electrolytic cell. Adv Mater Res 800:496–500. https://doi.org/10.4028/www.scientific.net/AMR.800.496
Hitachi Ltd (2010) Hitachi develops recycling technologies for rare earth metals. http://www.hitachi.com/New/cnews/101206.html. Accessed 27 Jul 2015
Mitsubishi Electric (2014) Environment—when will rare earth recycling start to become widespread? http://www.mitsubishielectric.com/company/environment/ecotopics/rareearth/when/index.html. Accessed 13 Jul 2015
Tsakalakis K, Stamboltzis GA (2004) Modeling the specific grinding energy and ball-mill scaleup. In: IFAC Conference 2004, Nancy
Tsakalakis K, Stamboltzis GA (2004) Modelling the specific grinding energy and ball-mill scaleup: presented at 11th symposium on automation in MMM IFAC 2004, Nancy, France
Talens Peiró L, Villalba Méndez G (2013) Material and energy requirement for rare earth production. JOM 65(10):1327–1340. https://doi.org/10.1007/s11837-013-0719-8
Yamamura T, Mehmood M, Maekawa H et al (2004) Electrochemical processing of rare earth and rare earth metals by using molten salts. Chem Sust Dev 12:105–111
Gunawan A (2015) Thermodynamic considerations in molten salt electrolysis for rare earth metals, paper 22. Graduate Theses & Non-Theses, Montana Tech Library
Zhu H (ed) (2014) Rare earth metal production by molten salt electrolysis: encyclopedia of applied electrochemistry, pp 1765–1772
Vogel H, Flerus B, Stoffner F et al (2016) Reducing greenhouse gas emission from the neodymium oxide electrolysis. Part I: analysis of the anodic gas formation: thematic section: green rare earth element innovations in ore processing, hydrometallurgy, and electrolysis. J Sust Metall 3:99–107
Zhang Z, Liang X, Ju J, Xu G (2000) The current situation and latest progress of the preparation of Nd in fluoride system by Nd oxide electrolysis. Translated from Chinese by Wang J. In: Chinese Society of Rare Earth—Conference Proceedings, pp 207–211
Althaus H-J, Chudacoff M, Hischier R et al (2007) Life cycle inventories of chemicals: ecoinvent report no 8. Data V2.0, Dübendorf
The Aluminium Association (2013) The environmental footprint of semi-finished aluminum products in North America: a life cycle assessment report, http://aluminum.org/sites/default/files/LCA_Report_Aluminum_Association_12_13.pdf. Accessed 18th May 2018
Wong DS, Fraser P, Lavoie P et al (2015) PFC emissions from detected versus nondetected anode effects in the aluminum industry. JOM 67:342. https://doi.org/10.1007/s11837-014-1265-8
Jamieson MB (2014) NETL life cycle inventory data process documentation file: rare earth oxide electrolysis. Electrolysis of a rare earth oxide using a fluoride-based process. Brief description
Schreiber A, Marx J, Zapp P et al (2016) Environmental impacts of rare earth mining and separation based on eudialyte: a new European way. Resources 5(4):32. https://doi.org/10.3390/resources5040032
Zaimes GG, Hubler BJ, Wang S, Khanna V (2015) Environmental life cycle perspective on rare earth oxide production. ACS Sustain Chem Eng 3:237–244. https://doi.org/10.1021/sc500573b
Navarro J, Zhao F (2014) Life-cycle assessment of the production of rare-earth elements for energy applications: a review. Front Energy Res 3(45):1–17. https://doi.org/10.3389/fenrg.2014.00045
Schulze R, Weidema BP, Schebek L, Buchert M (2018) Recycling and its effects on joint production systems and the environment—the case of rare earth magnet recycling—part I—production model. Resour Conserv Recycl 134:336–346. https://doi.org/10.1016/j.resconrec.2017.11.006
IPCC (2013) Climate change 2013: the physical science basis. Contribution of Working Group I to the fifth assessment report of the intergovernmental panel on climate change. Stocker TF, Qin D, Plattner G-K, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM. University Press Cambridge, Cambridge
Kim J, Fraser PJ, Li S et al (2014) Quantifying aluminum and semiconductor industry perfluorocarbon emissions from atmospheric measurements. Geophys Res Lett 41(13):4787–4794. https://doi.org/10.1002/2014gl059783
Keller R, Larimer KT (1998) United States Patent—electrolytic production of neodymium without perfluorinated carbon compounds on the offgases (5,810,993). Accessed 19 Dec 2016
Keller R (1998) Proceedings of the symposium on energy and electrochemical processing for a cleaner environment: electrolytic production of neodymium—with and without emission of greenhouse gases. https://books.google.de/books?id=5Xy5jHSwgTwC&pg=PA143&lpg=PA143&dq=electrolytic+production+of+neodymium+keller+97-28&source=bl&ots=h2sXPTQSyh&sig=W84WSznQ80_8q8Uz0D3-lE5qsco&hl=de&sa=X&ved=0ahUKEwi70JaN34DRAhUEtBoKHVIxAN8Q6AEIHjAA#v=onepage&q=electrolytic%20production%20of%20neodymium%20keller%2097-28&f=false. Accessed 19 Dec 2016
Keniry J, Kjar AR (2013) Electrolytic cell for production of rare earth metals (WO 2013170299 A1; CA2879712A1, CN104520476A, EP2850226A1, EP2850226A4, US20150159286, WO2013170299A8)
Trudinger CM, Fraser PJ, Etheridge DM et al (2016) Atmospheric abundance and global emissions of perfluorocarbons CF4, C2F6 and C3F8 since 1800 inferred from ice core, firn, air archive and in situ measurements. Atmos Chem Phys 16:11733–11754
Li B, Liu S, Wang H et al (2014) Electrochemistry for Nd electrowinning from fluoride-oxide molten salts. In: Neelameggham NR, Alam S, Oosterhof H et al (eds) Rare metal technology 2014. Wiley, Hoboken, NJ, pp 95–98
MPC (2017) Massachusetts-produced rare earth metals: infinium’s new separation process promises cleaner, cheaper source for critical ingredient in hybrid/electric vehicles and less dependence on Chinese imports. Accessed 10 Jan 2017
Wang C, de Bakker J, Belt CK et al (2013) Energy technology 2014: carbon dioxide management and other technologies. Wiley, Hoboken
Mühle J, Ganesan AL, Miller BR et al (2010) Perfluorocarbons in the global atmosphere: tetrafluoromethane, hexafluoroethane, and octafluoropropane. Atmos Chem Phys 10(11):5145–5164. https://doi.org/10.5194/acp-10-5145-2010
Vogel H, Friedrich B (2018) An estimation of PFC emission by rare earth electrolysis. TMS annual meeting & exhibition TMS 2018: Light Metals 2018, pp 1507–1517
Cai et al (2018) Estimating perfluorocarbon emission factors for industrial rare earth metal electrolysis. Resour Conserv Recycl 136:315–323
Zhang et al (2018) Perfluorocarbon emissions from electrolytic reduction of rare earth metals in fluoride/oxide system. Atmos Pollut Control 9(2018):61–65
World Aluminium (2016) Fluoride emissions. http://www.world-aluminium.org/statistics/fluoride-emissions/. Accessed 10 Jan 2017
Paulin I, Donik C, Jenko M (2009) Mechanisms of HF bonding in dry scrubber in aluminium electrolysis. Mater. Technol. 43(4):189–193
JRC (2011) ILCD handbook—international reference life cycle data system: recommendations for life cycle impact assessment in the European context—based on excisting environmental impact assessment models and factors
Nuss P, Eckelman MJ, Janssen PJ (2014) Life cycle assessment of metals: a scientific synthesis. PLoS ONE 9(7):e101298. https://doi.org/10.1371/journal.pone.0101298
Gauß R, Diehl O, Brouwer E et al (2015) Verfahren zum recycling von seltenerdhaltigen Permanentmagneten. Chem Ing Tec 87(11):1477–1485. https://doi.org/10.1002/cite.201500061
Acknowledgements
The authors would like to thank Prakash Venkatesan, Enrique Herraiz, Mehmet Recai Önal, Konstantinos G. Tsakalakis, and Irena Skulj for help with the compilation of LCI data and/or discussions concerning the preparation of the EOL-magnet material; Yongxian Yang for discussions regarding the industrial-scale implementation of the electrolysis process; Xiaoling Guo for her help with the translation of Chinese language publications; and Bernd Friedrich, Ksenija Milicevic, and Hanno Vogel for a conversation on PFC emissions. The research leading to these results has received funding from the European Community’s Seventh Framework Programme [FP7/2007–2013] under Grant agreement No. 607411 (MC-ITN EREAN: European Rare Earth Magnet Recycling Network). This publication reflects only the authors’ views, exempting the Community from any liability. Project website: http://www.erean.eu.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest to disclose.
Additional information
The contributing editor for this article was Gabrielle Gaustad.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Schulze, R., Abbasalizadeh, A., Bulach, W. et al. An Ex-ante LCA Study of Rare Earth Extraction from NdFeB Magnet Scrap Using Molten Salt Electrolysis. J. Sustain. Metall. 4, 493–505 (2018). https://doi.org/10.1007/s40831-018-0198-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-018-0198-9