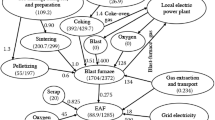
Abstract
This paper derives from first principles simple relationships that can be used to compute energy requirements for the production of hot metal (pig iron) in a blast furnace (BF) or direct reduced iron (DRI) in a direct reduction furnace (DRF), and the transformation of hot metal and DRI into crude steel in a basic oxygen furnace (BOF) or electric arc furnace (EAF) with the addition of scrap iron or scrap steel of varying purity. These relationships account for the impact of changing iron ore grade, in the amount and type of impurities in iron, and the impact of the addition of fluxes and the production of slag. Changing proportions of hot metal and scrap to the BOF, or of DRI and scrap to the EAF, are accounted for. The energy flow analysis presented here, combined with the mass flow analysis presented in a companion paper, provides a foundation for tracking the impact on energy use and iron losses of alternative pathways that might be used in the future as part of a broad-based effort to reduce energy use and associated greenhouse gas emissions.
Graphical Abstract





Similar content being viewed by others
Change history
13 May 2022
A Correction to this paper has been published: https://doi.org/10.1007/s40831-022-00539-3
Notes
Added limestone is converted to CaO in the blast furnace, a process called calcination.
Energy use associated with production of prepared iron as lumps, pellets and sinter is omitted here but will be considered in a follow-up paper that traces energy use from mining through to manufactured steel products.
Due to quantum–mechanical effects, specific heats increase rapidly from zero at 0 K, but still increase slowly with temperature as temperature increases beyond 300 K, asymptotically approaching the theoretical limit expected from the equipartition of vibrational energy. See Snow [7] for a more complete discussion.
For Cases 5 and 6, offgas otherwise available is consumed, so the offgas term adds to the net energy requirement.
Of which 2.11 GJ/t for the reaction enthalpy change, 1.30 GJ/t to heat the iron, and 5.28 GJ/t as feedstock energy.
Oxygen can be enriched to a concentration of up to 29% [8]. This reduces the required N2 and its heating, but requires energy for cryogenic separation of O2 from air. These opposing impacts on energy use are neglected here.
This is the fuel efficiency taking into account production of CO by reactions involving SiO2, MnO and CaCO3. Also shown in Table 7 are fuel efficiencies (which are higher) based solely on the iron-reduction reactions.
Precipitation of Fe3C in DRI as DRI forms occurs when the reacting gas contains > 20% CH4 with H2/CO ~ 5 [14].
Consumers of DRI want a minimum C content in DRI. Thus, Stena (https://www.stenametalinc.com/hot-briquetted-iron-hbi/hot-briquetted-iron-hbi) guarantees a minimum C content in their HBI product of 0.6%, with a typical C content of 0.7%. In considering production of DRI with H2 produced from C-free electricity sources in order to reduce CO2 emissions, Yilmaz and Turek [17] assumed continued use of a minimal amount of natural gas so as to provide 0.5% C in DRI. A greater amount of combustible C (see “Production of Steel in EAFs” section) results in a lower N content of the output steel by avoiding the need for anthracite or for injection of fine graphite (Kirschen et al. [5]).
The process can use fuels already consisting of a mixture of CO and H2.
Fruehan et al. [12] give the minimum theoretical energy to produce pure Fe DRI as 8.36 GJ/t, based on heating the ore to 900 °C and then reducing it. Here, the energy requirement computed this way is 8.37 GJ/t if the energy required to heat the CH4 fuel to the reaction temperature is ignored, or 8.72 GJ/t if it is included.
With increasing C content, the multiplier for R3 also decreases (as there is less CO from methane reforming available for absorption, due to the decrease in the R22 and R23 multipliers), but the R8 multiplier increases (to absorb the H2 produced by carburization).
For EAFs, Kirschen et al. [5] indicate efficiencies in transferring electrical and fuel energy inputs to the melt of 0.6–0.8 and 0.5–0.6, respectively.
The melting requirement increases with the scrap fraction because the added scrap is cold, while the hot metal is assumed to arrive with a fraction fhm of the required sensible heat gain. The melting energy also increases with the C fraction because the specific heat of C is greater than that of iron on a unit mass basis, and because more O2 must be heated (which also causes as slight increase in fCO).
As shown in the Electronic Supplementary Material (Sect. 4), the net effect of a cycle involving production of cementite when DRI is formed, and destruction of cementite when DRI is refined, is the reaction CH4 → C + 2H2 and a total reaction energy that is equal to that for this direct reaction. Electronic Supplementary Material Sect. 5 shows that the net reaction for reduction of SiO2 and MnO in the BF and re-oxidation in the BOF is the reaction nC + n/2O2 → nCO.
In these calculations, it is assumed that all the heat released from chemical reactions contributes to the EAF energy requirements. In reality, some portion would be lost as sensible heat in the offgas, but this would not affect the theoretical minimum energy requirement, as this assumes that all the offgas sensible heat (and chemical energy) is used in some way (so that it can be credited against the EAF energy requirement).
References
IIMA (International Iron Metallics Association) (n.d.) Use of direct reduced iron (DRI) in the electric arc furnace (EAF) for steelmaking. Burnham. www.metallics.org
UNFCC (United Nations Framework Convention on Climate Change) (2015) Paris agreement. https://unfccc.int/files/essential_background/convention/application/pdf/english_paris_agreement.pdf. Accessed 14 July 2019
Masson-Delmotte V, Zhai P, Pörtner H-O, Roberts D, Skea J, Shukla PR (eds) (2018) Global warming of 1.5 °C: an IPCC special report on the impacts of global warming of 1.5 °C above pre-industrial levels and related greenhouse gas emission pathways, in the context of strengthening the global response to the threat of climate change, sustainable development, and efforts to eradicate poverty. Summary for policymakers. Intergovernmental Panel on Climate Change, Geneva
Remus R, Monsonet MAA, Roudier S, Sancho LD (2010) Best available techniques (BAT) reference document for iron and steel production. European Commission, JRC Reference Report, Industrial Emissions Directive 2010/75/EU; 2013
Kirschen M, Badr K, Pfeifer H (2011) Influence of direct reduced iron on the energy balance of the electric arc furnaces in steel industry. Energy 36:6146–6155
Lüngen HB, Yagi J-I (2013) Iron, 2. Blast furnace process. In Ullmann’s encyclopedia of industrial chemistry. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim
Snow CLM (2010) The heat capacity and thermodynamic properties of the iron oxides and their relation to the mineral core of the iron storage protein ferritin. PhD thesis, Brigham Young University
Yang Y, Raipala K, Holappa L (2014) Chapter 1.1, Ironmaking. In: Seetharaman S (ed) Treatise on process metallurgy, volume 3: industrial processes. Elsevier, Amsterdam, pp 2–88
Lüngen HB (2018) Trends for reducing agents in blast furnace operation. Presented at 2nd German Polish symposium carbon materials for metal production, tradition and progress. Freiberg, Germany, 16 Oct 2013. https://www.dkg.de/akk-vortraege/2013-_-2rd_polnisch_deutsches_symposium/abstract-luengen_reducing-agents.pdf. Accessed 9 July 2018
De Beer J, Worrell E, Blok K (1998) Future technologies for energy-efficient iron and steel making. Ann Rev Energy Environ 23:123–205
JFE 21st Century Foundation (2013) An introduction to iron and steel processing, chapter 2, smelting, refining and continuous casting, 2D, BF operation. https://www.jfe-21st-cf.or.jp/chapter_2/2d_2_img.html. Accessed 5 Feb 2020.
Fruehan RJ, Fortini O, Paxton HW, Brindle R (2000) Theoretical minimum energies to produce steel for selected conditions. U.S. Department of Energy Office of Industrial Technologies, Washington
Bhattacharya A, Muthusamy S (2017) Static heat energy balance mathematical model for an iron blast furnace. Int J Min Process Extr Metall 2:57–67
Villa A (2016) Flexibility of the energiron technology in DR plants. 3rd India international DRI summit 2016. https://www.spongeironindia.in/6.%20Presentations%202016%20(attached)%209%20PPTs%20and%201%20Vedio%20in%20vediopresentatins%20segment/Andr%C3%A9s%20Villa.pdf. Accessed 7 April 2018
Morales J (2015) Economics with high-carbon DRI from ENERGIRON DR Technology. Middle east iron and steel conference. Dubai https://www.metalbulletin.com/events/download.ashx/document/speaker/7916/a0ID000000X0kGhMAJ/Presentation. Accessed 28 Mar 2018.
Morales J (2015) ENERGIRON—The innovative direct reduction technology. Iranian iron and steel conference. Kish Island. https://www.metalbulletin.com/events/download.ashx/document/speaker/7962/a0ID000000X0kHrMAJ/Presentation. Accessed 28 Mar 2018
Yilmaz C, Turek T (2017) Modeling and simulation of the use of direct reduced iron in a blast furnace to reduce carbon dioxide emissions. J Clean Prod 164:1519–1530
Formanek L, Rose F, Prölβ ( 2013) Iron, 3. Direct reduction processes. In: Ullmann’s encyclopedia of industrial chemistry. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim
Midrex (2014) The Midrex® process—the world’s most reliable and productive direct reduction technology. Charlotte, NC
Battle T, Srivastava U, Kopfle J, Hunter R, McCleland J (2014) Chapter 1.2, The direct reduction of iron. In: Seetharaman S (ed) Treatise on process metallurgy, volume 3: industrial processes. Elsevier, Amsterdam, pp 89–176
Cullen JM, Allwood JM, Bambach MD (2012) Mapping the global flow of steel: from steelmaking to end-use goods. Environ Sci Technol 46:13048–13055
Jalkanen H, Holappa L (2014) Chapter 1.4: Converter steelmaking. In: Seetharaman S (ed) Treatise on process metallurgy, volume 3: industrial processes. Elsevier, Amsterdam, pp 223–270
IEA (International Energy Agency) (2013) Iron and steel CCS Study (Techno-economics integrated steel mill). Report: 2013/04, July 2013. International Energy Agency, Paris
Lee B, Sohn I (2014) Review of innovative energy savings technology for the electric arc furnace. JOM 66(9):1581–1594. https://doi.org/10.1007/s11837-014-1092-y
Madias J (2014) Chapter 1.5, Electric furnace steelmaking. In: Seetharaman S (ed) Treatise on process metallurgy, volume 3: industrial processes, pp 271–300
Sohn HY (2008) Suspension hydrogen reduction of iron ore concentrate. American Iron and Steel Institute Technology Roadmap Program, Final Project Report
IEA (International Energy Agency) (2007) Tracking industrial energy efficiency and CO2 emissions. International Energy Agency, Paris
Energiron (2020) Energiron HYL, DRI Technology by Tenova and Danieli. https://www.energiron.com/wp-content/uploads/2020/01/Energiron_Brochure-2020.pdf. Accessed 5 Feb 2020. https://www.energiron.com/
Haupt M, Vadenbo C, Zeltner C, Hellweg S (2016) Influence of input-scrap quality on the environmental impact of secondary steel production. J Ind Ecol 21:391–401
Atkinson M, Kolarik R (2001) AISI Steel Industry Technology roadmap Report (American Iron and Steel Institute, Oak Ridge, TN). https://www.energy.gov/sites/prod/files/2013/11/f4/roadmap_chap2.pdf. Accessed 30 Mar 2018
Harvey LDD (2020) From iron ore to crude steel: mass flows associated with lumps, pellets, sinter and scrap iron inputs. ISIJ Int 60:1139–1151
Harvey LDD (2020) A model of the present-day iron and steel production process and energy use (in preparation)
Acknowledgements
I am indebted to Lauri Holappa (Aalto University, Finland) and one anonymous reviewer for their meticulous and thorough reviews of initial drafts of this paper, leading to significant improvements.
Funding
This research received no funding support in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
The contributing editor for this article was Sharif Jahanshahi.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised: The graphical abstract and Fig. 2 in this article as originally published omitted non-energy-containing offgases CO2 and H2O from the Direct Reduction Furnace.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Harvey, L.D.D. Analysis of the Theoretical and Practical Energy Requirements to Produce Iron and Steel, with Summary Equations that Can Be Applied in Developing Future Energy Scenarios. J. Sustain. Metall. 6, 307–332 (2020). https://doi.org/10.1007/s40831-020-00276-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-020-00276-5