Abstract
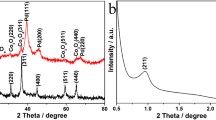
Two-dimensional (2D) mesoporous metal-oxide (hydroxide) nanomaterials with defects are promising towards the realization of efficient electrocatalysis. Herein, we report a facile and effective one-pot solvothermal route to synthesize mesoporous Mox-Co-O hybrid nanosheets (NSs) which is composed of crystalline Mo4O11 and amorphous cobalt hydroxide. Due to the corrosion of 1-octylamine at high temperatures, abundant mesoporous holes are created in situ over the Mox-Co-O hybrid NSs during the solvothermal process, which is beneficial to increasing the electrochemical surface area. The dimension of the Mox-Co-O NSs, size of mesoporous and the concentration of defects can be easily modulated by controlling the molar ratio of Mo/Co. Electrochemical measurements reveal that the 2D mesoporous Mox-Co-O NSs show an excellent activity for the oxygen evolution reaction with the highest catalytic activity of η10 = 276 mV at 10 mA cm−2 in 1 mol L−1 KOH. Enhanced adsorption of intermediates and abundant oxygen vacancies achieved by appropriate Mo doping are the two main factors that contribute to the excellent catalytic activity of Mo0.2-Co-O NSs. This work, with the construction of 2D metal-oxide (hydroxide) crystalline-amorphous nanomaterials possessing abundant holes, oxygen vacancies and enhanced adsorption of intermediates, provides important insight on the design of more efficient catalysts.

摘要
构建具有丰富缺陷且缺陷浓度可调的二维(2D)介孔金属氧化物 (氢氧化物)纳米材料作为高效电催化剂仍然是一个巨大的挑战. 本工作 开发了一种简便有效的一锅溶剂热路线来合成由晶体相Mo4O11和准非 晶相氢氧化钴组成的介孔Mox-Co-O杂化纳米片. 由于短链有机胺在高 温下的刻蚀作用, 合成过程中在Mox-Co-O杂化纳米片上原位产生了大 量的介孔空穴, 增加了材料的电化学比表面积. 此外, Mox-Co-O纳米片 的尺寸、介孔和晶相/非晶相纳米片上缺陷(本工作中的氧空位)的浓度 可以通过控制Mo/Co摩尔比调节. 电化学测试表明, 2D介孔Mox-Co-O 纳米片表现出优异的析氧反应活性, 其中Mo0.2-Co-O纳米片表现出最 高的催化活性(在1 mol L−1 KOH中10 mA cm−2处的过电位为276 mV). 研究发现, Mo0.2-Co-O纳米片具有合适的d带中心和最高浓度的氧空 位, 这是促成其优异催化活性的两个主要因素. 本工作构建具有丰富缺 陷(氧空位)和合适d带中心的二维金属氧化物(氢氧化物)的晶相/非晶 杂化纳米材料的方法, 为设计更高效的催化剂提供了重要启发.
Similar content being viewed by others
References
Larcher D, Tarascon JM. Towards greener and more sustainable batteries for electrical energy storage. Nat Chem, 2015, 7: 19–29
Stamenkovic VR, Strmcnik D, Lopes PP, et al. Energy and fuels from electrochemical interfaces. Nat Mater, 2017, 16: 57–69
King LA, Hubert MKA, Capuano C, et al. A non-precious metal hydrogen catalyst in a commercial polymer electrolyte membrane electrolyser. Nat Nanotechnol, 2019, 14: 1071–1074
Zhan W, Sun L, Han X. Recent progress on engineering highly efficient porous semiconductor photocatalysts derived from metal-organic frameworks. Nano-Micro Lett, 2019, 11: 1–3
You B, Sun Y. Innovative strategies for electrocatalytic water splitting. Acc Chem Res, 2018, 51: 1571–1580
Kibsgaard J, Chorkendorff I. Considerations for the scaling-up of water splitting catalysts. Nat Energy, 2019, 4: 430–433
Hunter BM, Gray HB, Müller AM. Earth-abundant heterogeneous water oxidation catalysts. Chem Rev, 2016, 116: 14120–14136
You B, Jiang N, Sheng M, et al. High-performance overall water splitting electrocatalysts derived from cobalt-based metal-organic frameworks. Chem Mater, 2015, 27: 7636–7642
You B, Zhang Y, Jiao Y, et al. Negative charging of transition-metal phosphides via strong electronic coupling for destabilization of alkaline water. Angew Chem Int Ed, 2019, 58: 11796–11800
Stoerzinger KA, Rao RR, Wang XR, et al. The role of Ru redox in pH-dependent oxygen evolution on rutile ruthenium dioxide surfaces. Chem, 2017, 2: 668–675
Xu X, Liang H, Ming F, et al. Prussian blue analogues derived penroseite (Ni,Co)Se2 nanocages anchored on 3D graphene aerogel for efficient water splitting. ACS Catal, 2017, 7: 6394–6399
Bai L, Duan Z, Wen X, et al. Highly dispersed ruthenium-based multifunctional electrocatalyst. ACS Catal, 2019, 9: 9897–9904
Oh HS, Nong HN, Reier T, et al. Electrochemical catalyst-support effects and their stabilizing role for IrOx nanoparticle catalysts during the oxygen evolution reaction. J Am Chem Soc, 2016, 138: 12552–12563
Shan J, Guo C, Zhu Y, et al. Charge-redistribution-enhanced nanocrystalline Ru@IrOx electrocatalysts for oxygen evolution in acidic media. Chem, 2019, 5: 445–459
Jayaramulu K, Masa J, Tomanec O, et al. Nanoporous nitrogen-doped graphene oxide/nickel sulfide composite sheets derived from a metal-organic framework as an efficient electrocatalyst for hydrogen and oxygen evolution. Adv Funct Mater, 2017, 27: 1700451
Nsanzimana JMV, Peng Y, Xu YY, et al. An efficient and earth-abundant oxygen-evolving electrocatalyst based on amorphous metal borides. Adv Energy Mater, 2018, 8: 1701475
Guo D, Chen F, Zhang W, et al. Phase-transfer synthesis of α-Co(OH)2 and its conversion to CoO for efficient electrocatalytic water oxidation. Sci Bull, 2017, 62: 626–632
Zhang B, Zhang J, Tan X, et al. One-step synthesis of ultrathin α-Co(OH)2 nanomeshes and their high electrocatalytic activity toward the oxygen evolution reaction. Chem Commun, 2018, 54: 4045–4048
Zheng S, Tu Q, Urban JJ, et al. Swelling of graphene oxide membranes in aqueous solution: Characterization of interlayer spacing and insight into water transport mechanisms. ACS Nano, 2017, 11: 6440–6450
Zhang X, Lai Z, Tan C, et al. Solution-processed two-dimensional MoS2 nanosheets: Preparation, hybridization, and applications. Angew Chem Int Ed, 2016, 55: 8816–8838
Zheng J, Zhang H, Dong S, et al. High yield exfoliation of two-dimensional chalcogenides using sodium naphthalenide. Nat Commun, 2014, 5: 2995
Yin Y, Han J, Zhang Y, et al. Contributions of phase, sulfur vacancies, and edges to the hydrogen evolution reaction catalytic activity of porous molybdenum disulfide nanosheets. J Am Chem Soc, 2016, 138: 7965–7972
Ullman AM, Brodsky CN, Li N, et al. Probing edge site reactivity of oxidic cobalt water oxidation catalysts. J Am Chem Soc, 2016, 138: 4229–4236
Jiang Y, Li X, Wang T, et al. Enhanced electrocatalytic oxygen evolution of α-Co(OH)2 nanosheets on carbon nanotube/polyimide films. Nanoscale, 2016, 8: 9667–9675
Jin H, Mao S, Zhan G, et al. Fe incorporated α-Co(OH)2 nanosheets with remarkably improved activity towards the oxygen evolution reaction. J Mater Chem A, 2017, 5: 1078–1084
Zhuang L, Ge L, Yang Y, et al. Ultrathin iron-cobalt oxide nanosheets with abundant oxygen vacancies for the oxygen evolution reaction. Adv Mater, 2017, 29: 1606793
Fominykh K, Tok GC, Zeller P, et al. Rock salt Ni/Co oxides with unusual nanoscale-stabilized composition as water splitting electrocatalysts. Adv Funct Mater, 2017, 27: 1605121
Zhang SL, Guan BY, Lu XF, et al. Metal atom-doped Co3O4 hierarchical nanoplates for electrocatalytic oxygen evolution. Adv Mater, 2020, 32: 2002235
Cai Z, Bi Y, Hu E, et al. Single-crystalline ultrathin Co3O4 nanosheets with massive vacancy defects for enhanced electrocatalysis. Adv Energy Mater, 2018, 8: 1701694
Osgood H, Devaguptapu SV, Xu H, et al. Transition metal (Fe, Co, Ni, and Mn) oxides for oxygen reduction and evolution bifunctional catalysts in alkaline media. Nano Today, 2016, 11: 601–625
Nai J, Yin H, You T, et al. Efficient electrocatalytic water oxidation by using amorphous Ni-Co double hydroxides nanocages. Adv Energy Mater, 2015, 5: 1401880
Cai P, Huang J, Chen J, et al. Oxygen-containing amorphous cobalt sulfide porous nanocubes as high-activity electrocatalysts for the oxygen evolution reaction in an alkaline/neutral medium. Angew Chem Int Ed, 2017, 56: 4858–4861
Li H, Chen S, Jia X, et al. Amorphous nickel-cobalt complexes hybridized with 1T-phase molybdenum disulfide via hydrazine-induced phase transformation for water splitting. Nat Commun, 2017, 8: 15377
Huang C, Wu S, Sanchez AM, et al. Lateral heterojunctions within monolayer MoSe2-WSe2 semiconductors. Nat Mater, 2014, 13: 1096–1101
Liu YC, Koza JA, Switzer JA. Conversion of electrodeposited Co(OH)2 to CoOOH and Co3O4, and comparison of their catalytic activity for the oxygen evolution reaction. Electrochim Acta, 2014, 140: 359–365
He X, Song X, Qiao W, et al. Phase- and size-dependent optical and magnetic properties of CoO nanoparticles. J Phys Chem C, 2015, 119: 9550–9559
Gao R, Liu L, Hu Z, et al. The role of oxygen vacancies in improving the performance of CoO as a bifunctional cathode catalyst for rechargeable Li-O2 batteries. J Mater Chem A, 2015, 3: 17598–17605
Cai L, McClellan CJ, Koh AL, et al. Rapid flame synthesis of atomically thin MoO3 down to monolayer thickness for effective hole doping of WSe2. Nano Lett, 2017, 17: 3854–3861
Diaz-Droguett DE, El Far R, Fuenzalida VM, et al. In situ-Raman studies on thermally induced structural changes of porous MoO3 prepared in vapor phase under He and H2. Mater Chem Phys, 2012, 134: 631–638
Patil MK, Gaikwad SH, Mukherjee SP. Phase- and morphology-controlled synthesis of tunable plasmonic MoO3−x nanomaterials for ultrasensitive surface-enhanced Raman spectroscopy detection. J Phys Chem C, 2020, 124: 21082–21093
Zhou Y, Dong CK, Han L, et al. Top-down preparation of active cobalt oxide catalyst. ACS Catal, 2016, 6: 6699–6703
Huang K, Sun Y, Zhang Y, et al. Hollow-structured metal oxides as oxygen-related catalysts. Adv Mater, 2019, 31: 1801430
Nguyën HC, Garcés-Pineda FA, de Fez-Febré M, et al. Non-redox doping boosts oxygen evolution electrocatalysis on hematite. Chem Sci, 2020, 11: 2464–2471
Wang H, Wang J, Pi Y, et al. Double perovskite LaFexNi1−xO3 nanorods enable efficient oxygen evolution electrocatalysis. Angew Chem Int Ed, 2019, 58: 2316–2320
He D, Song X, Li W, et al. Active electron density modulation of Co3O4-based catalysts enhances their oxygen evolution performance. Angew Chem Int Ed, 2020, 59: 6929–6935
Acknowledgements
This work was financially supported by the National Key R&D Program of China (2020YFB1505802), the Ministry of Science and Technology (2017YFA0208200), the National Natural Science Foundation of China (22025108, U21A20327, and 22121001), and the Start-up Funds from Xiamen University.
Author information
Authors and Affiliations
Contributions
Huang X conceived and supervised the research. Huang X and He C designed the experiments. He C, Hu X, Wang J, Bu L, Zhan C and Xu B performed most of the experiments and data analysis. Huang X, Li L, He C and Li Y wrote the paper. All authors discussed the results and commented on the manuscript.
Corresponding authors
Additional information
Conflict of interest
The authors declare that they have no conflict of interest.
Supplementary information
Supporting data are available in the online version of the paper.
Chuansheng He received his PhD degree (2021) in physical chemistry from Beijing Normal University under the supervision of Prof. Yunchao Li. He is currently a postdoctoral fellow in Prof. Xiaoqing Huang’s group at Xiamen University. His research focuses on the design of nanomaterials and the exploration of the structure-activity relationship between nanomaterials and the electrocatalytic performance.
Leigang Li received his PhD degree in materials science and engineering from Texas A&M University under the supervision of Prof. Haiyan Wang in 2017. He then worked as a postdoctoral research fellow at Purdue University. Since 2018, he has been working as a postdoctoral research fellow in Prof. Xiaoqing Huang’s group. His current research interest focuses on the design and synthesis of metallic nanostructures for electrocatalytic applications.
Xiaoqing Huang is currently a professor at the College of Chemistry and Chemical Engineering, Xiamen University. He obtained his BSc degree in chemistry education from the Southwest Normal University (2005) and PhD degree in inorganic chemistry from Xiamen University (2011) under the supervision of Profs. Nanfeng Zheng and Lansun Zheng. He then worked as a postdoctoral research associate in Profs. Yu Huang and Xiangfeng Duan’s group from 2011 to 2014 at the University of California, Los Angeles. His current research interest focuses on the design and synthesis of nanomaterials for energy-related applications such as electrocatalysis and photocatalysis.
Supporting Information for
40843_2022_2098_MOESM1_ESM.pdf
Defect engineered 2D mesoporous Mo-Co-O nanosheets with crystalline-amorphous composite structure for efficient oxygen evolution
Rights and permissions
About this article
Cite this article
He, C., Hu, X., Wang, J. et al. Defect engineered 2D mesoporous Mo-Co-O nanosheets with crystalline-amorphous composite structure for efficient oxygen evolution. Sci. China Mater. 65, 3470–3478 (2022). https://doi.org/10.1007/s40843-022-2098-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-022-2098-x