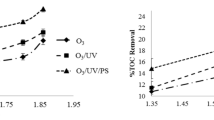
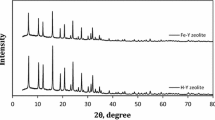
Abstract
Advanced oxidation processes (AOPs) gain attention for wastewater treatment due to the formation of hydroxyl radicals, which have more oxidation potential. Among all AOPs, few O3, O3/UV, O3/UV/persulfate (PS), and O3/catalyst processes were studied to degrade RB5 dye wastewater. Furthermore, the effect of various experimental parameters like ozone flowrate (30–60 LPH), initial pH (2–12), initial dye concentration (100–1000 mg/L), UV intensity (11–66 W), persulfate dosage, and catalyst dosage (0.5–1.2 g/L) was studied for degradation of RB5. Furthermore, the prepared catalyst was characterized by X-ray diffraction, scanning electron microscopy, energy-dispersive spectroscopy, and BET surface area. Based on the results obtained in the study, the maximum TOC removal efficiency was 96% achieved with optimum operating parameters, 60 LPH of ozone flowrate, 7 pH, 100 mg/L RB5 concentration, and 1 g/L catalyst dosage in 80 min of reaction time using O3/catalyst process, while in O3/UV/PS process, the total organic carbon (TOC) removal efficiency was 90% with optimum operating parameters, 60 LPH of ozone flowrate, 12 pH, 100 mg/L RB5 concentration, UV intensity 66 W, and TOC/PS ratio 1:40 in 80 min of reaction time. Finally, it can be seen that ozone-based AOPs offered an effective solution for the degradation of recalcitrant pollutants, especially RB5.









Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this article.
References
Jose A, Lara-Ramos S-G, Daniel V-R, Jennyfer D-A, Mueses M, Machuca-Martínez F (2019) Intensification of the O3/TiO2/UV advanced oxidation process using a modified flotation cell. Photochem Photobiol Sci 18:920–928. https://doi.org/10.1039/C8PP00308D
Ambaye TG, Hagos K (2020) Photocatalytic and biological oxidation treatment of real textile wastewater. Nanotechnol Environ Eng 5(3):1–11
Baruah M, Supong A, Bhomick PC, Karmaker R, Pongener C, Sinha D (2020) Batch sorption–photodegradation of Alizarin Red S using synthesized TiO 2/activated carbon nanocomposite: an experimental study and computer modelling. Nanotechnol Environ Eng. https://doi.org/10.1007/s41204-020-00071-3
Dhiman P, Kumar A, Shekh M, Sharma G, Rana G, Vo DVN, ALOthman ZA (2021) Robust magnetic ZnO-Fe2O3 Z-scheme hetereojunctions with in-built metal-redox for high performance photo-degradation of sulfamethoxazole and electrochemical dopamine detection. Environ Res 197:111074
Dhiman P, Rana G, Kumar A, Sharma G, Vo DVN, AlGarni TS, ALOthman ZA (2021) Nanostructured magnetic inverse spinel Ni-Zn ferrite as environmental friendly visible light driven photo-degradation of levofloxacin. Chem Eng Res Des. https://doi.org/10.1016/j.cherd.2021.08.028
Dhiman P, Patial M, Kumar A, Alam M, Naushad M, Sharma G, Kumar R (2021) Environmental friendly and robust Mg0. 5-xCuxZn0. 5Fe2O4 spinel nanoparticles for visible light driven degradation of Carbamazepine: band shift driven by dopants. Mater Lett 284:129005
Kumar J, Bansal A (2015) CFD simulations of immobilized-titanium dioxide based annular photocatalytic reactor: Model development and experimental validation. Indian J Chem Technol 22:95–104
Ratan JK, Saini A (2019) Enhancement of photocatalytic activity of self-cleaning cement. Mater Lett 244:178–181
Saini A, Arora I, Ratan JK (2020) Photo-induced hydrophilicity of microsized-TiO2 based self-cleaning cement. Mater Lett 260:126888
Zhou X, Meng M, Sun Z, Li Q, Jiang Z (2011) Prominent enhancement of Mn or Co addition on the performance of Cu-Ce-O catalyst used for H2 production via dimethyl ether steam reforming. Chem Eng J 174:400–407. https://doi.org/10.1016/j.cej.2011.09.018
Zhang N, Li JM, Liu GG, Chen XL, Jiang K (2017) Photodegradation of diclofenac in seawater by simulated sunlight irradiation: the comprehensive effect of nitrate, Fe(III) and chloride. Mar Pollut Bull 117(1–2):386–391. https://doi.org/10.1016/j.marpolbul.2017.02.024
Zhou X, Meng M, Sun Z, Li Q, Jiang Z (2011) Prominent enhancement of Mn or Co addition on the performance of Cu-Ce-O catalyst used for H2 production via dimethyl ether steam reforming. Chem Eng J 174(1):400–407
Mecha AC, Onyango MS, Ochieng A, Fourie CJS, Momba MNB (2016) Synergistic effect of UV–vis and solar photocatalytic ozonation on the degradation of phenol in municipal wastewater: a comparative study. J Catal 341:116–125. https://doi.org/10.1016/j.jcat.2016.06.015
Parrino F, Camera-Roda G, Loddo V, Palmisano G, Augugliaro V (2014) Combination of ozonation and photocatalysis for purification of aqueous effluents containing formic acid as probe pollutant and bromide ion. Water Res 50:189–199. https://doi.org/10.1016/j.watres.2013.12.001
Hussain M, Mahtab MS, Farooqi IH (2020) The applications of ozone-based advanced oxidation processes for wastewater treatment: a review. Adv Environ Res 9(3):191–214. https://doi.org/10.12989/aer.2020.9.3.191
Amr A, Salem S, Aziz HA, Adlan MN, Bashir MJK (2013) Pretreatment of stabilized leachate using ozone/persulfate oxidation process. Chem Eng J 221:492–499. https://doi.org/10.1016/j.cej.2013.02.038
Hilles AH, AbyAmr SS, Alkarkhi AFM, Hossain MS (2019) The effect of persulfate oxidation on the biodegradability of concentrated anaerobic stabilized leachate. Sains Malays 48(11):2381–2390. https://doi.org/10.17576/jsm-2019-4811-09
Soubh A, Mokhtarani N (2016) The post treatment of composting leachate with a combination of ozone and persulfate oxidation processes. RSC Adv 80:76113–76122. https://doi.org/10.1039/C6RA09539A
Cao J, Zhang W-X, Brown DG, Sethi D (2008) Oxidation of lindane with Fe(II)-activated sodium persulfate. Environ Eng Sci 25(2):221–228. https://doi.org/10.1089/ees.2006.0244
Lin Y-T, Liang C, Chen J-H (2011) Feasibility study of ultraviolet activated persulfate oxidation of phenol. Chemosphere 82(8):1168–1172. https://doi.org/10.1016/j.chemosphere.2010.12.027
Soubh AM, Baghdadi M, Abdoli MA, Aminzadeh B (2018) Activation of persulfate using an industrial iron-rich sludge as an efficient nanocatalyst for landfill leachate treatment. Catalysts 8:218. https://doi.org/10.3390/catal8050218
Li P, Miao R, Wang P, Sun F, Li X-Y (2021) Bi-metal oxide-modified flat-sheet ceramic membranes for catalytic ozonation of organic pollutants in wastewater treatment. Chem Eng J. https://doi.org/10.1016/j.cej.2021.131263
Guo L, Xiao Z, Sun W, Hao Xu, Yanhua Xu, Zheng H, Sun Y (2021) Fischer–Tropsch synthetic wastewater treatment with Fe/Mn@CH: catalytic ozonation and process evaluation. Sep Purif Technol 276:119274. https://doi.org/10.1016/j.seppur.2021.119274
Beltran FJ, Rivas FJ, Montero-de-Espinosa R (2003) Ozone-enhanced oxidation of oxalic acid in water with cobalt catalysts. 2. Heterogeneous catalytic ozonation. Ind Eng Chem Res 42:3218–3224
Faria PCC, Monteiro DCM, Órfão JJM, Pereira MFR (2009) Cerium, manganese and cobalt oxides as catalysts for the ozonation of selected organic compounds. Chemosphere 74:818–824. https://doi.org/10.1016/j.chemosphere.2008.10.016
Ghuge SP, Saroha AK (2018) Catalytic ozonation for the treatment of synthetic and industrial effluents—application of mesoporous materials: a review. J Environ Manag 211:83–102. https://doi.org/10.1016/j.jenvman.2018.01.052
Zhang H, He Y, Lai L, Yao G, Lai Bo (2020) Catalytic ozonation of Bisphenol A in aqueous solution by Fe3O4–MnO2 magnetic composites: performance, transformation pathways and mechanism. Sep Purif Technol 245:116449. https://doi.org/10.1016/j.seppur.2019.116449
Hu E, Wu X, Shang S, Tao X-M, Jiang S-X, Gan L (2016) Catalytic ozonation of simulated textile dyeing wastewater using mesoporous carbon aerogel supported copper oxide catalyst. J Clean Prod 112:4710–4718. https://doi.org/10.1016/j.jclepro.2015.06.127
Cuerda-Correa EM, Alexandre-Franco MF, Fernández-González C (2020) Advanced oxidation processes for the removal of antibiotics from water. An overview. Water. https://doi.org/10.3390/w12010102
Furman OS, Teel AMYL, Watts RJ (2010) Mechanism of base activation of persulfate. Environ Sci Technol 44:6423–6428. https://doi.org/10.1021/es1013714
Liang C, Guo YY (2012) Remediation of diesel-contaminated soils using persulfate under alkaline condition. Water Air Soil Pollut 223:4605–4614. https://doi.org/10.1007/s11270-012-1221-6
Wong CPP, Lai CW, Lee KM, Hamid SBA (2015) Advanced chemical reduction of reduced graphene oxide and its photocatalytic activity in degrading reactive black 5. Material 8(10):7118–7128. https://doi.org/10.3390/ma8105363
Chokshi NP, Patel D, Atkotiya R, Ruparelia J (2020a) Catalytic ozonation of Reactive Black 5 in aqueous solution over a La-Co-O catalyst. J Indian Chem Soc 97:373–378
Zhu H, Ma W, Han H, Han Y, Ma W (2017) Catalytic ozonation of quinoline using nano-MgO: efficacy, pathways, mechanisms and its application to real biologically pretreated coalgasification wastewater. Chem Eng J. https://doi.org/10.1016/j.cej.2017.06.025
Ren Y, Dong Q, Feng J, Ma J, Wen Q, Zhang M (2012) Magnetic porous ferrospinel NiFe2O4: a novel ozonation catalyst with strongcatalytic property for degradation of di-n-butyl phthalate and convenientseparation from water. J Colloid Interface Sci 382:90–96. https://doi.org/10.1016/j.jcis.2012.05.053
Lovato ME, Gilliard MB, Cassano AE, Martín CA (2014) Kinetics of the degradation of n-butyl benzyl phthalate using O3/UV, direct photolysis, direct ozonation and UVeffects. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-014-2796-9
Paucar NE, Kim I, Tanaka H, Sato C (2019) Effect of O3 dose on the O3/UV treatment process for the removal of pharmaceuticals and personal care products in secondary effluent. ChemEngineering. https://doi.org/10.3390/chemengineering3020053
Rekhate CV, Srivastava JK (2020) Recent advances in ozone-based advanced oxidation processes for treatment of wastewater: a review. Chem Eng J Adv 3:100031. https://doi.org/10.1016/j.ceja.2020.100031
Yang S, Yang X, Shao X, Niu R, Wang L (2011) Activated carbon catalyzed persulfate oxidation of Azo dye acid orange 7 at ambient temperature. J Hazard Mater 186:659–666. https://doi.org/10.1016/j.jhazmat.2010.11.057
Deng J, Shao Y, Gao N, Deng Y, Tan C, Zhou S (2014) Zero-valent iron/persulfate(Fe0/PS) oxidation acetaminophen in water. Int J Environ Sci Technol 11:881–890. https://doi.org/10.1007/s13762-013-0284-2
Chokshi NP, Ruparelia JP (2021) Synthesis of nano Ag-La-Co composite metal oxide for degradation of RB 5 dye using catalytic ozonation process. Ozone Sci Eng. https://doi.org/10.1080/01919512.2021.1901070
Ali F, Khan JA, Shah NS, Sayed M, Khan HM (2018) Carbamazepine degradation by UV and UV-assisted AOPs: kinetics, mechanism and toxicity investigations. Process Saf Environ Prot 117:307–314. https://doi.org/10.1016/j.psep.2018.05.004
Chokshi NP, Ruparelia JP (2020b) Catalytic ozonation of Reactive Black 5 over silver-cobalt composite oxide catalyst. J Inst Eng India Ser A 101:433–443. https://doi.org/10.1007/s40030-020-00454-4
Mena E, Rey A, Beltrán FJ (2018) TiO2 photocatalytic oxidation of a mixture of emerging contaminants: a kinetic study independent of radiation absorption based on thedirect-indirect model. Chem Eng J 339:369–380. https://doi.org/10.1016/j.cej.2018.01.122
Lara-Ramos JA, Saez C, Machuca-Martínez F, Rodrigo MA (2020) Electro-ozonizers: a new approach for an old problem. Sep Purif Technol 241:116701. https://doi.org/10.1016/j.seppur.2020.116701
Liu Z, Demeestere K, Van Hulle S (2021) Comparison and performance assessment of ozone-based AOPs in view of trace organic contaminants abatement in water and wastewater: a review. J Environ Chem Eng 9:105599. https://doi.org/10.1016/j.jece.2021.105599
Acknowledgements
The authors would like to thank Nirma University, Ahmedabad, Gujarat, for providing all necessary help and support to carry out the research work.
Funding
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Consent for publication
Authors give copyrights to the journal as per the rules.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sharma, S., Chokshi, N.P. & Ruparelia, J.P. Comparative studies for the degradation of Reactive Black 5 dye employing ozone-based AOPs. Nanotechnol. Environ. Eng. 7, 745–752 (2022). https://doi.org/10.1007/s41204-021-00180-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41204-021-00180-7