Abstract
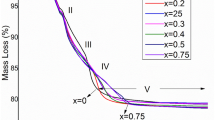
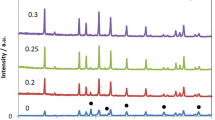
In this work, we have successfully synthesized a pure phase of α-alum KAl(SO4)2∙12H2O, denoted as KAlSD by the slow evaporation method, to be useful as a material in the storage energy domain. XRD analysis and IR spectroscopy confirmed the high pure cubic phase. Thermal dehydration results done by DTA-TG analysis confirmed a complete dehydration process around 520 K. Kinetic analysis using the linear regression analysis indicated that different models can explain each dehydration stage. The conduction mechanism process was followed by complex impedance spectroscopy (CIS). Conductivity within the material is thermally activated and occurs by the migration of mobile cations into the host lattice with activation energy equal to 1.08 eV. KAlSD is an ionic semiconductor that can be selected as good electrode material in the energy storage applications.













Similar content being viewed by others
Data Availability
The dataset used during this study is available from the corresponding author on resonnable request.
References
Xue L, Yan L, Xu X, Liang Z, Liqiang M (2021) Rechargeable metal (Li, Na, Mg, Al)-sulfur batteries: materials and advances. J Energy Chem 61:104–134
Dajian L, Yuan Y, Jiawei L, Maximilian F, Fusheng P (2021) A review on current anode materials for rechargeable Mg batteries. J Magnes Alloy 8:963–979
Liuzhang O, Jianling H, Hui W, Jiangwebg L, Min Z (2017) Progress of hydrogen storage alloys for Ni-MH rechargeable power batteries in electric vehicles: a review. Mater Chem Phys 200:164–178
Wenxu S, Wentao Y, Xu X, Yanyi M, Peng T, Meng N (2021) Unravel the influences of Ni substitution on Co-based electrodes for rechargeable alkaline Zn–Co batterie. J Power Sourc 483:229192
Ivana H, Sathiya M, Damien S, Philipp A, Alexey YK, Christian M, Laurence C, Montse CC (2021) Challenges of today for Na-based batteries of the future: From materials to cell metrics. J Power Sourc 482:228872
Caixia L, Chun CH, Liyu C, Stefan K, Qiang X (2021) Rechargeable Al-ion batteries. Energy Chem 3:100049
Giuseppe AE, Kostiantyn VK, Maksym VK, Joaqin C, Alex H, Richard GAW (2021) An overview and perspective on Al and Al-ion battery technologies. J Power Sourc 481:228870
Yu Z, Lei M, Ruixian T, Xiao Z, Xiaoyo W, Yanru D, Guolong K, Fangfang Z, Liangming W (2021) The host hollow carbon nanospheres as cathode material for nonaqueous room-temperature Al–S batteries. Int J Hydrog Energy 46:4936–4946
Jin TR, Lei C, Hao YW, Zhong YY (2021) Aqueous Al-N2 battery assembled by hollow molybdenum phosphate microspheres for simultaneous NH3 production and power generation. Chem Eng 418:129447
Guanghua L, Deshuang Y, Junnan S, Feng H, Linlin L, Shengjie P (2021) FeNi nanoparticles encapsulated in Nitrogen-doped carbon frame for efficient and stable Al-air batteries. Mater Lett 296:129890
Bo H, Liangxing J, Ketao H, Fangyang L, Xiaoying Y, Haitao X, Jie L, Yexiang L (2014) Al/Pb lightweight grids prepared by molten salt electroless plating for application in lead-acid batteries. J Power Sourc 256:294–300
Li Z, Quanhu M, Gaowei W, Ziqiang L, Li Z (2021) A low cost electrolyte of AlCl3/AcAm ionic liquid analogs for high-performance aluminum ion batteries. J Electroanal Chem 888:115176
Hanyan X, Tianwen B, Hao C, Fan G, Jiabin X, Tieqi H, Shengying C, Xingyan C, Jun L, Weiwei G, Zhen X, Chao G (2019) Low-cost AlCl3/Et3NHCl electrolyte for high-performance aluminum-ion battery. Energy Storage Mater 17:38–45
Ahmed S, Inocencio RM, Lotfi Z, Antonio DLG, Dalila BHC (2017) Luminescence properties of Pr3+ ion doped Mg-picromerite Tutton salt. J Lumin 188:148–153
Ahmed S, Massoud K, BenMoumammed M, Antonio DLG, Dalila BHC (2017) Synthesis, structural and electrochemical properties of new ytterbium doped langbeinite ceramics. Ceram Int 43:10939–10947
Ahmed S, Antonio DLG, Lotfi Z, Sameh AE, Mohammed A, Emmanuel L, Carla PR, Dalila BHC (2016) Synthesis, characterization and electrical properties of both pure and cobalt-doped picroerite-type hydrated double salt K2Mg1-xCox(SO4)2.6H2O (x = 0, 0.4). J Electron Mater 45:4418–4424
Abir B, Ahmed S, Massoud K, Antonio DLG, Dalila BHC (2018) Synthesis, electrical and dielectrical properties of (LixNa1– x)6Mg(SO4)4 vanthoffite ceramics as new attractive electrode materials for Li- and Na-ion batteries. Mater Sci Eng B 228:224–233
Abir B, Ahmed S, Massoud K, Antonio DLG, Julian MP, Dalila BHC (2019) Synthesis, characterization, thermal analysis and electrical properties of (NH4)2M(SO4)2·6H2O (M=Cu Co, Ni). Mater Sci Eng B 240:97–105
Abir B, Ahmed S, Massoud K, Antonio DLG, Dalila BHC (2019) Spectroscopic investigations on vanthoffite ceramics partially doped with cobalt. Ionics 24:2867–2875
Ahmed S, Massoud K, Ramzi F, Antonio DLG, Dalila BHC (2019) Synthesis, characterization and electrical properties of Na6M(SO4)4 (M=Co, Ni, Cu) vanthoffite materials. Mater Sci Eng B 244:56–64
Ahmed S, Massoud K, Inocencio RM, Antonio DLG, Emmanuel L, Dalila BHC (2019) Synthesis, luminescence, and electrical properties of Na6Mg(SO4)4:xEu vanthoffite ceramics as electrode materials for sodium ion batteries. Mater Sci Eng B 247:114384
Zheng M, Luo JW, Zhang YH, Chen P (2017) Preparation and characterization of composite material Na2SO4·10H2O-KAl(SO4)2·12H2O for thermal storage. Mater Sci Eng 167:012007
Jianfeng D, Runqiang L, Kezhan W (2012) Thermal performance and dehydration kinetics of KAl(SO4)2·12H2O as phase change material. Adv Mater Res 418–420:282–285
Kaminskii AA, Haussuhl E, Haussuhl S, Eichler HJ, Ueda K, Hanuza J, Takaichi K, Rhee H, Gad GMA (2004) α−Alums: K, Rb, Tl and NH4Al(SO4)2.12H2O—a new family of χ3−active crystalline materials for Raman laser converters with large frequency shifts. Laser Phys Lett 1:205–211
Nyburg SC, Steed JW, Aleksovska S, Petrusevski VM (2000) Structure of the alums. I. On the sulfate group disorder in the α−alums. Acta Cryst B 56:204–209
Gad G, Kaminskii AA, Eichler HJ, Pickardt J (2003) Double sulfate KAl(SO4)2.12H2O single crystals (a-alum)—a new material for Raman laser converters. Phys Stat Sol A 3:R10–R12
Ko JH, Oh SH, Lee KS (2020) Anomalous longitudinal acoustic phonon and elastic constant in potassium alum KAl(SO4)2⋅12H2O single crystal studied by Brillouin spectroscopy. J Phys Chem Solids 140:109364
Ae RL, Se YJ (2004) A study of the relaxation mechanism of a KAl(SO4)2·12H2O single crystal by observation of its 39K and 27Al spin–lattice relaxation processes. J Phys: Condens Matter 16:4403–4410
Wunchun S, Yan Z, Jinxin F, Xiaoming F, Ziye L, Zhengguo Z (2019) Compounding MgCl2.6H2O with NH4Al(SO4)2.12H2O or KAl(SO4)2.12H2O to obtain binary hydrated salts as high-performance phase change materials. Molecules 24:363
Ae RL (2009) Study on the phase transitions by nuclear magnetic resonance of α-type RbAl(SO4)2.12H2O and β−type CsAl(SO4)2.12H2O single crystals. Solid State Nucl Magn Reson 36:45–51
Paolo B (2015) Thermal behaviour of alum-(K) KAl(SO4)2·12H2O from in situ laboratory high-temperature powder X-ray diffraction data: thermal expansion and modelling of the sulfate orientational disorder. Mineral Mag 79:157–170
Goutam B, Khondekar N, Sanchari B, Mullicka M, Nayana N, Indrajit K, Mandal B (2019) Alum (KAl(SO4)2.12H2O)—an eco-friendly and versatile acid-catalyst in organic transformations: a recent update. Curr Green Chem 6:12–31
Minoo D, Peyman S, Somayeh O, Mostafa BGK, Ali AM (2005) Efficient synthesis of mono- and disubstituted 2,3-dihydroquinazolin-4(1H)-ones using KAl(SO4)2.12H2O as a reusable catalyst in water and ethanol. Tetrahedron Lett 46:6123–6126
Majid MH, Hossein AO, Narges K, Hoda H (2011) KAl(SO4)2.12H2O catalyzed efficient synthesis of 3,4,6-trisubstituted 2-pyridone in water. Chin Chem Lett 22:1059–1062
Majid MH, Masoumeh Z, Narges M, Hoda H (2012) KAl(SO4)2.12H2O or KHSO4: efficient and inexpensive catalysts for the one-pot synthesis of β-acetamido ketones by dakin-west reaction. Synth React Inorg M 42:178–182
Kiran FS, Suryakant S, Amol K, Bapu S, Murlidhar S (2009) An efficient and green procedure for the preparation of acylals from aldehydes catalyzed by alum [KAl(SO4)2.12H2O]. S Afr J Chem 62:109–112
Brian HT (2006) R factors in rietveld analysis: how good is good enough? Powder Diff 21:67–70
Marine R, Prabeer B, Gwenaelle R, Jean NC, Brent CM, Nadir R, Jean MT (2012) Synthesis and crystal chemistry of the NaMSO4F family (M=Mg, Fe Co, Cu, Zn). Solid State Sci 14:15–20
Shubham L, Sudhindra MA, Lalit SP (2022) Magnetic structure of fluorophosphate Na2MnPO4F sodium battery material. J Solid State Chem 308:122926
Hamdi BY, Rachid E, Ruhul A, Khalid B, Toyoki O, Ilias B (2018) Sodium intercalation in the phosphosulfate cathode NaFe2(PO4)(SO4)2. J Power Sourc 382:144–151
Reza EK, Mohammed HA (2008) Evaluation of reliability of Coats-Redfern method for Kinetic analysis of non isothermal TGA. Trans Nonferrous Met Soc Chin 18:217–221
Luis APM, Criado JM, Sánchez PE (2006) Combined kinetic analysis of solid-state reactions: a powerful tool for the simultaneous determination of kinetic parameters and the kinetic model without previous assumptions on the reaction mechanism. J Phys Chem A 110:12456–12462
Sergey V, Alain KB, Jose MC, Luis APM, Crisan P, Nicolas S (2011) ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta 520:1–19
Nicolas S (2019) Advanced isoconversional kinetic analysis for the elucidation of complex reaction mechanisms: a new method for the identification of rate-limiting steps. Molecules 24:1683
Ahmed S, Dalila BHC (2021) Effects of Gd2O3 doping on the structure and the conduction mechanism of K2Mg2(SO4)3 langeinite ceramics: a comparative study. Mater Sci Eng B 265:115040
Amor F, Salah K, Hassouna D (2018) BixCe1−xPO4(x = 0.00, 0.02, and 0.08) nanorods: structural, electrical, optical, and electrochemical properties. Ionics 24:429–450
Acknowledgements
The authors would like to thank Mrs. Jmii Imen the Thermal Analysis Laboratory technician for her help, suggestions, and comments during the experimental procedure. We are also grateful to Mr. Othmani Abdelhak for his help in impedance measurements.
Funding
The authors received no financial support conflict of interest concerning the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declared no potential conflict of interest concerning the research, authorship, and/or publication of this article.
Rights and permissions
About this article
Cite this article
Souemti, A., Mouhammed, M.B., Lozano-Gorrin, A.D. et al. Investigations on KAl(SO4)2∙12H2O: A Candidate α-Alum Material for Energy Storage Applications. Chemistry Africa 5, 575–587 (2022). https://doi.org/10.1007/s42250-022-00336-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42250-022-00336-1