Abstract
The present study aims to know the groundwater hydrochemistry and its drinking suitability in Kadava river basin through Water Quality Index (WQI) modelling approach. Eighty (80) dug/bore well samples were collected and analysed for the span of dry (pre) and wet (post) monsoon seasons of 2012 by following standard procedures of APHA. According to BIS standard, the parameters, viz., pH, Ca, Mg, Na, Cl, NO3, TH and TDS, surpass the threshold limits in both the seasons. It is observed that TDS content in pre- (75%) and post (97.5%)-monsoon seasons is exceeding the desirable limit (500 mg/l). Also, 67.5% (pre-monsoon) and 75% (post-monsoon) of the groundwater samples are beyond the nitrate permissible limit (45 mg/l) of the BIS. The magnesium content in 15 and 37.5% samples is surpassing the permissible limit (100 mg/l) in pre- and post-monsoon seasons. TH content in 15 and 25% samples surpassed the permissible limit (600 mg/l) in pre- and post-monsoon seasons. WQI results demonstrated that 50.0 and 22.5% samples come under good quality of water; 47.5 and 72.5% samples fall in poor water category, while 2.5 and 5.0% samples exhibit very poor category in pre-/post-monsoon seasons. Hydrochemical characterization based on Durov plot, Chaddha diagram, Gibbs plot and various scatter plots was used to ascertain the types of weathering, influence of rock, precipitation and evaporation, types of reactions, etc., influenced on groundwater composition of the region. The groundwater chemistry is mostly deteriorated due to the influence of agricultural and anthropogenic activities. Spatial variation map of WQI exhibits that groundwater quality is affected mainly in south and few patches in north regions of the study area.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Groundwater is one of the reliable and vital sources of drinking water because of its widespread availability, occurring in natural condition and less susceptible to water contamination as compared to fresh water [1,2,3]. Many studies proved that in developing countries, scarcity of clean and safe drinking water is inauspiciously affecting the public health and related health issues [4]. Usually, groundwater quality is control by natural processes precipitation, rock–water interaction, residence time of water, dissolution of salts and its mineralization, rock weathering like silicate and carbonate, water percolation, leaching of salts and anthropogenic inputs are alter the water quality [5,6,7]. In India, groundwater quality is deteriorated due to overexploitation without balanced recharge; use of surplus amount of agrochemicals and fertilizers percolates into the aquifer systems [8,9,10,11]. Thus, it is imperative to know that the hydrochemical behaviour, contaminant sources and continuous monitoring of groundwater quality become need of hour in arid and semi-arid regions for maintaining the water quality in natural state.
The assessment of large number of monitored parameters turns out to be difficult to evaluate the exact nature of groundwater quality and to represent it as single unit [12,13,14]. The aptness of groundwater quality depends upon amount of cleanliness and load of pollution [15, 16]. Horton has developed the Water Quality Index by considering, ranking and integrating the significant physico-chemical parameters [17]. Afterwards, Brown et al. [18] have developed the improved version of Horton WQI known as NSF WQI model.
In the Kadava river basin, groundwater is mainly used to fulfil the needs of drinking, domestic and agriculture; therefore, groundwater quality is intimately linked with human health. Yet, very limited research was carried out on the groundwater quality and its suitability; nonetheless, studies carried out by CGWB and individual reported high amount of nitrate and heavy metals in groundwater [19,20,21,22,23,24]. Consequently, continuous monitoring and evaluation of water quality are essential to avoid further groundwater contamination problems which affect human health. The objective of the present study was (1) to ascertain the hydrochemistry and groundwater suitability in Kadava river basin through WQI model, (2) to find out the controlling processes which influence the hydrochemistry of the region and (3) to prepare the spatial distribution maps to know the spatial extent of water quality deterioration in the study area.
2 Study area
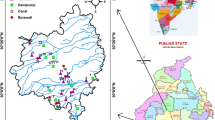
The Kadava river basin lies in Nashik District, Maharashtra, (latitude 19°55′ and 20°25′N; longitude 73°55 and 74°15′E) with an area of 1053 km2 (Fig. 1). The river has its origin in Sahyadri hills near to Dindori in Nashik district. The Kadava River flows into northwest to southeast direction and confluence to the Godavari river in Niphad Tehsil. The average rainfall of the study area is around 700 mm spanning over June–September mainly from SW monsoonal winds with semi-arid climate, and temperature varies 5–40 °C in winter and summer seasons [19]. The area distinguished part of Deccan Plateau, and basalt is the dominant rock from upper Cretaceous to lower Eocene age and comprising ‘Pahoehoe’ and ‘Aa’ lava flows [25]. The agricultural land in the downstream part of the Kadava basin is mainly drained through Kadava river, Palkhed canal and groundwater, and dominant crops like sugarcane, grapes, onion and other crops such as vegetables, flowers and fruits as subsidiary are cultivated throughout the year. Apart from the irrigation use, groundwater is also used as major source to cater the drinking water demand because of the scarcity of central water supply systems in this area.
3 Materials and methods
To know the hydrochemical behaviour of groundwater, eighty (80) water samples were collected from dug/bore wells during pre- and post-monsoon seasons of 2012. The samples were collected in pre-treated (1 l) plastic cans; prior to the sample collection each sampled well discharge water for 2–3 min to reduce the influence of stored pipe water and transported to laboratory for physico-chemical analysis. pH and EC were measured in situ by handheld multi-parameter tester. Major cations, viz., Ca, Mg, Na and K, and anions CO3, HCO3, SO4, NO3 and Cl were analysed by following the standard procedures of American Public Health Association [26]. The ion balance errors are within (± 5%) which confirm the analytical accuracy (Table 1).
3.1 Conceptualization of Water Quality Index (WQI)
WQI is used to measure the combined influence of individual physico-chemical parameters on the whole quality of water for drinking [17, 27]. The WQI provides a single numerical value to assess the quality of water based on the results of pH, EC, Cl, NO3, Na, K, CO3, HCO3, TDS, TH, Ca, Mg and SO4. The Bureau of Indian Standards (BIS) for drinking was considered for calculation of WQI index [28]. The weightage was assigned to each individual parameters based on the perceived threat to water quality. The weights were assigned on scale of 1–5 based on their importance in drinking and threatening potential to human health [29,30,31,32]. The maximum weight 5 was assigned to TDS, Cl and NO3 due to their major importance in drinking suitability. The minimum weightage of 2 was assigned to Mg, K, CO3, Ca and HCO3, while rest of the parameters (pH, Na, SO4, TH and EC) were assigned with weight 3. The relative weight (RWi) of each parameter calculated with following equation is represented in Table 2. Based on these mentioned techniques, WQI for the groundwater of Kadava river basin has been studied and values are represented in Table 4. WQI is classified into five categories, i.e. excellent type: 0–50; good: 50–100; poor: 100–200; very poor: 200–300; and unfit for drinking: > 300.
where Rwi is relative weight and Awi are assigned weight of ith parameters.
The quality rating scale for water quality variables is expressed by the following equation
where qi is quality rating for ith parameter, ci is content of ith chemical parameter of water sample (mg/l), and si is permissible standard for ith parameter set by the BIS.
Sub-index (SIi) for each parameter is calculated by Eq. 3
where SIi is sub-index of ith parameter and qi is rating based on concentration of ith parameter.
Finally, WQI is computed by Eq. 4
4 Results and discussion
The hydrochemical results are assembled into three groups, namely Group I: pH, EC and TDS; Group II: cations; Group III: anions and their statistical summary are presented in Table 3. Also, the results of WQI and their spatial variability are interpreted. The governing processes controlling groundwater chemistry based on Durov plot, Chaddha diagram, Gibbs plot and various scatter plots used to ascertain the types of weathering, influence of rock, precipitation and evaporation, types of reactions, etc., are discussed.
4.1 Group I: pH, EC and TDS
The pH has no direct effect on human heath, but it can alter the taste of water and exhibit associations with some other water quality variables [33]. The pH value varied from 7.80 to 9.00 and 7.70 to 8.50 with an average 8.50 and 8.10 in pre- and post-monsoon seasons (Table 1) showing alkaline nature; the increased values of pH are attributed to the weathered basalt. The spatial variation maps of pH demonstrated that the samples, i.e. 9, 10, 11, 12 and 13, are located at north direction, where pH value is increased. Also, in pre-monsoon season the south, east, west and central parts have high pH value. It is confirmed that the sample locations 21, 24, 29, 34 and 36 are observed as hot spots in the study area (Fig. 2a, b). The conductivity of water is very significant because it increases in temperature and total dissolved salts [34]. EC shows a wide range, i.e. from 810 to 6180 µS/cm (avg. 2174 µS/cm) and 824 to 8120 µS/cm (avg. 2741 µS/cm) in pre-/post-monsoon seasons. The spatial variation maps of EC explore that sample numbers 20, 33, 37 and 38 of south and 7, 8, 10 and 11 locations of north regions show same spatial pattern in both the seasons (Fig. 2c, d). The TDS is an essential parameter used to confirm the suitability of drinking and irrigation water. TDS values varied from 357 to 2273 mg/l and 494 to 2102 mg/l with average values of (740 and 861 mg/l) in pre- and post-monsoon seasons, respectively (Table 1). It is demonstrated that 75 in pre- and 97.5% in post-monsoon seasons exceeded the desirable limit (Table 1), while sample number 38 surpassed permissible limit (2000 mg/l). Such high TDS in groundwater is contributed from soil salts, rock weathering, prolonged residence time and local anthropogenic inputs [35].
4.2 Group II: cations
In the study area, calcium content varies from 13.6 to 98.1 mg/l (avg. 39.8 mg/l) and 21.0 to 118 mg/l (avg. 50.9 mg/l) during pre- and post-monsoon seasons, respectively (Table 1). As compared the analytical results with BIS, all the samples are within threshold limit, thus suitable for drinking. The calcium content in the pre-monsoon season gets enriched due to more interaction with host rock as hydraulic gradient is low [36]. The magnesium ranges from 20.9 to 231 mg/l and 17.4 to 256 mg/l, and 15 and 37.5% samples are beyond the permissible limit of BIS. The high magnesium is exhibited from Thakurwadi formation; with high MgO content, contains picrite basalt and picrite with phenocrysts of olivine and clinopyroxene [37]. The high magnesium in post-monsoon is contributed by picritic flows of Thakurwadi formation which gets reduced in summer with ceased rainfall [36]. The spatio-temporal maps of magnesium illustrated that the south and north regions of the study area contain high concentration of magnesium. The magnesium hot spots are identified in intense agricultural zone in pre- and post-monsoon seasons. The high content of magnesium is observed in sample location 8, 10, 11, 27, 37, 38 and 39 (Fig. 2e, f). The total hardness values deviate from 132 to 1029 mg/l and 197 to 1150 mg/l, whereas average is 392 and 509 mg/l in pre- and post-seasons. Analytical results corroborate that 62.5% and 92.5% samples exceeded the desirable limit (300 mg/l) and 15 and 25% samples surpassed the permissible limit (600 mg/l) in pre-/post-monsoon season (Table 1). Water hardness mainly influenced by multivalent ions dissolved from minerals [38]. Drinking water with high TH content may lead to urolithiasis and cardiovascular disorders [39]. The sodium content varies from 36.2 to 501 mg/l (avg. 111 mg/l) and 31.0 to 449 mg/l (avg. 114 mg/l). The sample numbers 20, 37, 38 and 39 depict that sodium concentration might have been increased due to the hydro-morphological conditions and groundwater flow path which exceeds the permissible limit in both the seasons. The sodium shows high solubility nature and source in groundwater from rock weathering and evaporation dominance [40]. The consumption of sodium content drinking water may lead to cardiac, renal and circulatory disorders [41]. The spatial distribution maps of sodium confirm that the south portion contains high sodium content in both the seasons due to rock weathering or dissolution of soil salts in groundwater (Fig. 2g, h). The potassium content ranges from 0.300 to 8.30 mg/l (avg. 2.49 mg/l) and 0.500 to 14.4 mg/l with average value of 2.52 mg/l (Table 1). It is depicted that all the samples are within threshold limit (12.0 mg/l) except sample number 9, located in agricultural area; consequently, the source may be potassium-rich fertilizers.
4.3 Group III: anions
The chloride value varies from 31.2 to 1086 mg/l (avg. 210 mg/l) and 63.3 to 830 mg/l (avg. 230 mg/l) (Table 1). It is observed that the average content of chloride is elevated in post-monsoon season owing to influence of percolation, leaching, infiltration and dissolution processes. The weathering of halite mineral, domestic waste, fertilizers, septic tanks, landfills sites are main cause of chloride content in groundwater [42, 43]. According to BIS standards, 27.5 and 32.5% samples exceeded the DL (250 mg/l) in pre- and post-monsoon samples, respectively. However, sample no. 38 surpassed the PL (1000 mg/l) in pre-monsoon season due to inputs from agricultural and domestic waste (Table 1). The excess content of chloride in drinking water can change the taste, corrosion and palatability of water [44]. The sulphate content varies from 31.5 to 261 mg/l, 49.7 to 360 mg/l with an average 112 and 175 mg/l in pre- and post-monsoon seasons, respectively; also, and all the samples are within permissible limit of the BIS (Table 1). The average content of sulphate is increased in post-monsoon season due to the percolation of sulphate-rich water from agricultural runoff. According to BIS standards, 67.5 and 75% groundwater samples exceeded the BIS PL (45.0 mg/l) (Table 1), which confirmed the incongruity for drinking. The nitrates mainly attributed from domestic waste, animal wastages, nitrogen-rich fertilizers, etc., [45, 46]. The consumption of nitrate-rich water leads to methaemoglobinaemia and affects the oxygen circulation in blood. Also, it causes the blue-baby syndrome in infants, hypertension and stomach cancer [47]. The spatial variation maps of nitrate reveal that high content of nitrate was observed in the north, central and west parts where intense agriculture prevails in both the seasons; also average content was found in post-monsoon season (Fig. 2i, j). The carbonates and bicarbonates in the groundwater are due to influence of silicate rock weathering, dissolution of carbonate precipitates and atmospheric and soil CO2 gas [48]. The bicarbonate concentration ranges from 20.0 to 250 mg/l (avg. 99.0 mg/l) and 30.0 to 300 mg/l (avg. 121 mg/l) in pre-/post-monsoon season (Table 1). The increased bicarbonate concentration in post-monsoon signifies the supply of CO2 from precipitation recharge and rock–water interaction [49, 50].
4.4 Water Quality Index (WQI)
The Water Quality Index is classified into five categories, viz., 0–50 is excellent type; 50–100: good; 100–200: poor; 200–300: very poor; and > 300 as unfit for drinking (Table 4). The WQI value varies from 63.3 to 278 and 77.6 to 289 in pre-/post-monsoon season. The average value indicates that overall water quality of basin is poor in both the seasons (pre-monsoon 113 and post-monsoon 127). Table 4 depicts that 50 and 22.5% samples have good quality of water, while 47.5 and 72.5% samples fall in poor water category in pre- and post-monsoon season, respectively. The study area is free from industrialization and dense urbanization, but still it is observed that 2.50% samples in pre-monsoon and 5.00% samples in post-monsoon represent very poor water quality which may be due to the prolonged intensive agriculture and consumption of poor quality water may affect human health. The groundwater quality in post-monsoon season is negatively affected from recharging water as it soak up the salts from agricultural and domestic waste. The overall groundwater quality is affected due to low hydraulic gradient and rock–water interaction. Figure 3 illustrates that many groundwater samples fall in good type of water; few samples from poor type and two samples (number 37 and 38) come under very poor type. The sample numbers (32 and 39) show progressive affinity of groundwater towards very poor category.
4.5 Spatial variation of WQI
The spatial variation map of WQI (Fig. 4) demonstrated the water quality for each location and confirmed its suitability for drinking. It is observed that the groundwater quality is found to be good category in the north and central part, while it turns dramatically to poor type in post-monsoon season. It is observed that sample numbers 8, 10 and 11 are located in highland and due to rainwater recharge the rock–water interaction increased which alters the groundwater quality in post-monsoon season. The sample number 27 which has poor quality in pre-monsoon turns to good in post-monsoon; it may be due the dilution from recharge water. The samples located along flow direction in south region (sample numbers 20, 32, 33, 37, 38 and 39) are identified as hot spots due the deteriorated water quality in both the seasons. This area is under continuous irrigation with predominant crops, viz., sugarcane, grapes, onion, which requires fertilizers regularly leading to the enrichment of nitrates and chlorides in surface water and groundwater. It is exhibited that many of the groundwater locations are found vulnerable and should be restricted for drinking use except central and north parts. The heterogeneity in groundwater quality in the entire basin is due to changing cropping pattern, monotonous crops, rainfall influence, weathering of rock and evaporation dominance etc.
5 Processes governing groundwater chemistry
To know the processes governing the hydrochemical behaviour of groundwater in the study area, it is essential to study the geological composition of rocks, natural and anthropogenic inputs, dissolution of rocks and minerals, precipitation and climate condition that influence the hydrochemistry in the study area. In general, during groundwater recharge, it reacts with soil, rock–water interactions; various types of weathering and significant anthropogenic inputs may amend groundwater chemistry [5]. In this study, hydrochemical characterization based on Durov plot, Chaddha Plot, Gibbs plot and various scatter plots was used to ascertain the types of weathering, influence of rock, precipitation and evaporation, types of reactions, etc., influenced on groundwater composition of the region.
5.1 Durov plot
Durov introduced this diagram which helps to identify the hydrochemical facies, water types and geochemical processes to evaluate the groundwater quality in the region [51]. It is composed of diagrams of two ternary diagrams of cations and anions plotted against each other in AquaChem software. Durov plot (Fig. 5a, b) for groundwater samples of Kadava river basin indicates that most of the samples are in the phase of mixing, dissolution with few in reverse ion exchange.
5.2 Chadha plot
Chadha diagram is a modified version of the Piper plot and the expanded Durov diagram [52]. It represents square or rectangular field which describes the overall ion distribution and groundwater characteristics for indicating of geochemical classification and hydrochemical processes of groundwater. The difference in mill equivalent percentage between alkaline earths (Ca + Mg) and alkali metals (Na + K), expressed as percentage reacting values, is plotted on the X axis, and the difference in mill equivalent percentage between weak acidic anions (CO3 + HCO3) and strong acidic anions (Cl + SO4) is plotted on the Y axis. The mill equivalent percentage differences between alkaline earths and alkali metals, and between weak acidic anions and strong acidic anions, would plot in one of the four possible sub-fields of the proposed diagram. This diagram is classified into eight groups which represent different water types. The results of Chadha diagram (Fig. 6a, b) represent that majority of the samples exceeds alkaline earths (field 1), while 2 and 3 samples exceed alkali metals over alkaline earths in both the seasons. It also represents the dominance of strong acids in excess of the weak acids except few samples in both the seasons. These results are supported by the Piper plot, indicating the alkaline earths and strong acid dominance in both the years due to the interaction between the alkaline earths and alkali metals that originate from soil or rock interactions with strong acidic anions and weak acidic anions in groundwater. So the combination of these two diagrams would be appropriate to demonstrate the hydrochemical facies present in groundwater of Kadava river basin.
5.3 Gibbs plot
Gibbs plot (Fig. 7a, b) is used to know the association of groundwater composition with the aquifer lithology and represent the source of chemical constituents in groundwater such as precipitation, rock and evaporation dominance [53]. The ratios for cations and anions, i.e. Na/(Na + Ca) and Cl–/(Cl + HCO3) and of the groundwater samples when plotted against relative values of TDS showed the evaporation dominance process in most of the samples from pre- and post-monsoon seasons of 2012. This behavioural pattern confirms that many of the groundwater samples from vicinity of agricultural area and evaporation make salinity increase by increase in Na and Cl contents with relation to the increase in TDS. In addition, anthropogenic inputs like agricultural fertilizers, mixed canal/river water and irrigation return flows also influence the evaporation by the increase in Na+ and Cl−, and thus, TDS is increased. This clearly demonstrates that apart from the natural source, artificial factor, namely anthropogenic activity, decides and dominates the change in chemical composition of groundwater [54]. The area under investigation is characterized as semi-arid region, where rainfall was less than average of 700 mm in 2011 and 2012; thus, evaporation process becomes dominant in controlling the groundwater chemistry.
5.4 Scatter plots
The Na/Cl ratio (Fig. 8a) is used to know the salinity mechanism in arid and semi-arid environment [55]. The halite maintains the ratio to 1 by releasing Na and Cl ions [56]. The ratio indicates good correlation in both the seasons and suggests that the possible source of Na is silicate weathering and whether ratio less than 1 indicates the possibility of ion exchanges of Na with ca and Mg in clay particles [57]. Scatter plot of Ca + Mg versus Cl (Fig. 8b) indicates moderate to good correlation in pre- and post-monsoon seasons. The plot signifies that the increase in salinity is caused by chloride and is associated with the increase in the contents of Ca + Mg possibly pointing to the process of ion exchange reaction [58]. The plot of HCO3 versus Na + K (Fig. 8c) shows weak correlation in both the seasons due to less contribution of ions from felsic lithologies, and it confirms orthoclase being least weathered mineral in the study area. The plot of HCO3 + Cl versus Cl (Fig. 8d) shows the positive correlation (r2 = 0.97, 0.95) in pre- and post-monsoon seasons which pointed out the influence of salinization due to agricultural activities. The plot of TZ + versus Cl (Fig. 8e) represents the positive correlation (r2 = 0.92 and 0.93) in pre- and post-monsoon seasons. In the study area, chloride content is enriched due to agricultural inputs such as use of excessive chemical fertilizers, irrigation water runoff, percolation and leaching of waste, etc. Ca + Mg versus HCO3 + SO4 (Fig. 8f) plot shows that all the samples are plotted below the equiline in both the seasons. This indicates that carbonate weathering and silicate weathering are the dominant processes controlling groundwater chemistry within area [59]. Scatter plot Ca + Mg versus TZ+ illustrated positive association in both the seasons and confirmed that most of the groundwater samples fall below the equiline and reflecting high contents of sodium and potassium with increasing dissolved solids (Fig. 8g). Scatter plot of Na/Cl versus Cl (Fig. 8h) inferred weak correlation in both the seasons because chloride associated with increase in the content of Ca + Mg and decrease in Na/Cl may be from ion exchange process which adsorbs sodium taking place into aquifer in exchange for calcium and magnesium [58]. The logarithmic bivariate plot of HCO3/Na versus Ca/Na (Fig. 8i) and Mg/Na versus Ca/Na (Fig. 8j) is used to know the type of weathering influencing the hydrochemistry of the study area. Generally, the higher solubility of sodium than calcium increases the sodium concentration; therefore, Ca/Na ratio is expected to be low which infers the silicate weathering as dominant geochemical process [60]. Figure 8i shows silicate weathering prevailing in this area; however, few samples in both the seasons point towards carbonate weathering as secondary weathering process. The presence of lime kankar supports carbonate weathering in the study area. It is confirmed that silicate weathering is the main hydrogeochemical process followed by carbonate weathering and ion exchange process which affect the groundwater quality of the study area.
6 Conclusions
This study incorporates the governing processes of hydrochemistry and WQI method to ascertain the groundwater suitability for drinking. Hydrochemical results indicate that the parameters, viz., pH, Ca, Mg, Na, Cl, NO3, TH and TDS, eventually deteriorating groundwater quality exceeded the desirable and permissible limits described by the BIS. Hydrochemical analysis inferred that groundwater is alkaline and possesses permanent hardness. The increased EC is ascription from salt dissolution and inorganic pollution load in the groundwater. The elevated content of Cl, NO3 and TDS is owed from intensive agriculture and local anthropogenic inputs. It is observed that the groundwater quality is significantly affected in post-monsoon season due to persuade of agricultural runoff, excessive application of chemical fertilizers, percolation and dissolution of salts in aquifer. WQI results inferred that 50.0 and 22.5%, 47.5 and 72.5%, 2.50 and 5.00% samples come under good, poor and very poor categories, respectively. The groundwater quality is significantly influenced in southern portion owed by anthropogenic inputs mainly from agriculture, while northern area is affected by geogenic and anthropogenic sources. WQI maps exhibit the category of water to demonstrate the good, marginal and vulnerable sites for effective management of water resources. Durov plot indicates that most of the samples are in the phase of mixing; dissolution with few in reverse ion exchange reactions is dominant. Gibbs diagram suggests that evaporation process becomes dominant in controlling the groundwater chemistry because area is characterized as arid and semi-arid region. Chadha plot suggests that majority of the samples exceeds alkaline earths, while few exceed alkali metals over alkaline earths in both the seasons. Also, it represents the dominance of strong acids in excess of the weak acids except few samples in both the seasons. Ca + Mg versus Cl plot signifies that salinity is increased because Cl is associated with the increase in the contents of Ca + Mg possibly pointing to the process of ion exchange reaction. The plot of HCO3 + Cl versus Cl pointed out the influence of salinity due to agricultural activities. Ca + Mg versus HCO3 + SO4 indicate that carbonate weathering and silicate weathering are the dominant processes controlling groundwater chemistry within area. The hydrochemical interpretation advocates that silicate weathering is the main hydrogeochemical process followed by carbonate weathering and ion exchange process which affect the groundwater chemistry of the studied area.
The outcomes of the study may help local governing bodies to identify the suitable and vulnerable sites for effective management of groundwater resources in the river basin.
References
Todd DK, Mays LW (2005) Groundwater hydrology, edition, vol 1625. Wiley, Hoboken
Wagh VM, Panaskar DB, Varade AM, Mukate SV, Gaikwad SK, Pawar RS, Muley AA, Aamalawar ML (2016) Major ion chemistry and quality assessment of the groundwater resources of Nanded tehsil, a part of southeast Deccan Volcanic Province, Maharashtra, India. Environ Earth Sci 75(21):1418
Varade AM, Yenkie RO, Shende RR, Golekar RB, Wagh VM, Khandare HW (2018) Assessment of water quality for the groundwater resources of urbanized part of the Nagpur District, Maharashtra (India). Am J Water Resour 6(3):89–111
Adimalla N (2018) Groundwater quality for drinking and irrigation purposes and potential health risks assessment: a case study from semi-arid region of South India. Expo Health 11(2):109–123
Todd DK (1980) Groundwater hydrology, 2nd edn. Wiley, New York
Paul R, Prasanna MV, Gantayat RR (2019) Groundwater quality assessment in Jirania Block, west district of Tripura, India, using hydrogeochemical fingerprints. SN Appl Sci 1:1055. https://doi.org/10.1007/s42452-019-1092-1
Mukate SV, Panaskar DB, Wagh VM, Baker SJ (2019) Understanding the influence of industrial and agricultural land uses on groundwater quality in semiarid region of Solapur, India. Environ Dev Sustain. https://doi.org/10.1007/s10668-019-00342-3
Goyal SK, Chaudhary BS, Singh O, Sethi GK, Thakur PK (2010) GIS based spatial distribution mapping and suitability evaluation of groundwater quality for domestic and agricultural purpose in Kaithal district, Haryana state, India. Environ Earth Sci 61(8):1587–1597
Wagh VM, Panaskar DB, Muley AA, Mukate SV, Lolage YP, Aamalawar ML (2016) Prediction of groundwater suitability for irrigation using artificial neural network model: a case study of Nanded tehsil, Maharashtra, India. Model Earth Syst Environ 2(4):196
Wagh VM, Panaskar DB, Muley AA, Mukate SV (2017) Groundwater suitability evaluation by CCME WQI model for Kadava river basin, Nashik, Maharashtra, India. Model Earth Syst Environ 3(2):557–565
Panaskar DB, Wagh VM, Muley AA, Mukate SV, Pawar RS, Aamalawar ML (2016) Evaluating groundwater suitability for the domestic, irrigation, and industrial purposes in Nanded Tehsil, Maharashtra, India, using GIS and statistics. Arab J Geosci 9(13):615
Lumb A, Halliwell D, Sharma T (2006) Application of the CCME water quality index to monitor water quality: a case study of the Mackenzie River basin, Canada. Environ Monit Assess 113:411–429
Sánchez E, Colmenarejo MF, Vicente J, Rubio A, García MG, Travieso L, Borja R (2007) Use of the water quality index and dissolved oxygen deficit as simple indicators of watersheds pollution. Ecol Indic 7(2):315–328. https://doi.org/10.1016/j.ecolind.2006.02.005
Cong LW, Bahadori A, Zhang J, Ahmad Z (2019) Prediction of Water Quality Index (WQI) using support vector machine (SVM) and least square-support vector machine (LS-SVM). Int J River Basin Manag. https://doi.org/10.1080/15715124.2019.1628030
Ott WR (1978) Water quality indices: a survey of indices used in the United States, EPA—600\4-78-005. Environmental Protection Agency, Washington
Steinhart CE, Schierow LJ, Chesters G (1981) A review of water quality and related indices. Great Lakes Environmental Planning Study contribution No. 38, Water Resources Centre, University of Wisconsin, Madison
Horton RK (1965) An index number system for rating water quality. J Water Pollut Control Fed 37(3):300–306
Brown RM, Mclelland NI, Deininger RA, Tozer RG (1970) A water quality index—Do we dare? Water Sew Works 117(10):339–343
CGWB (2014) Central groundwater report of Nashik. http://cgwb.gov.in/district_profile/maharashtra/nashik.pdf
Wagh VM, Panaskar DB, Muley AA (2017) Estimation of nitrate concentration in groundwater of Kadava river basin-Nashik district, Maharashtra, India by using artificial neural network model. Model Earth Syst Environ 3(1):36
Wagh VM, Panaskar DB (2017) Assessment of groundwater geochemistry with special reference to impact of intensive agricultural practices from part of Kadava river basin, District Nashik, Maharashtra. Unpublished Ph.D. Thesis
Wagh VM, Panaskar DB, Mukate SV, Gaikwad SK, Muley AA, Varade AM (2018) Health risk assessment of heavy metal contamination in groundwater of Kadava river basin, Nashik, India. Model Earth Syst Environ 4(3):969–980
Wagh V, Panaskar D, Muley A, Mukate S, Gaikwad S (2018) Neural network modelling for nitrate concentration in groundwater of Kadava river basin, Nashik, Maharashtra, India. Groundw Sustain Dev. https://doi.org/10.1016/j.gsd.2017.12.012
Wagh VM, Panaskar DB, Mukate SV, Aamalawar ML, Sahu UL (2018) Nitrate associated health risks from groundwater of Kadava river basin Nashik, Maharashtra, India. Hum Ecol Risk Assess Int J. https://doi.org/10.1080/10807039.2018.1528861
Geological Survey of India (GSI) (2001) District resource map. GSI, Nashik District
APHA (2005) Standard methods for the examination of water and wastewater, 21st edn. New York
Mitra BK, Sasaki C, Keijirou E (2006) Spatial and temporal variation of ground water quality in sand dune area of Aomori Prefecture in Japan. In: ASAE Annual Meeting American Society of Agricultural and Biological Engineers, p 1
BIS (2012) Indian standards specification for drinking water, BIS: 10500: 2012. http://www.cgwb.gov.in/Documents/WQ-standards.pdf
Vasant W, Dipak P, Aniket M, Ranjitsinh P, Shrikant M, Nitin D, Manesh A, Abhay V (2016) GIS and statistical approach to assess the groundwater quality of Nanded Tehsil, (MS) India. In: Proceedings of first international conference on information and communication technology for intelligent systems, vol 1. Springer, Berlin, pp 409–417
Wagh V, Panaskar D, Mukate S, Lolage Y, Muley A (2017) Groundwater quality evaluation by physicochemical characterization and water quality index for Nanded Tehsil, Maharashtra, India. In: Proceedings of the 7th international conference on biology, environment and chemistry, IPCBEE, vol 98
Kadam AK, Wagh VM, Muley AA, Umrikar BN, Sankhua RN (2019) Prediction of water quality index using artificial neural network and multiple linear regression modelling approach in Shivganga River basin, India. Model Earth Syst Environ 1–12
Kumar B, Singh UK, Ojha SN (2019) Evaluation of geochemical data of Yamuna River using WQI and multivariate statistical analyses: a case study. Int J River Basin Manag 17(2):143–155
Islam ARMT, Ahmed N, Bodrud-Doza M, Chu R (2017) Characterizing groundwater quality ranks for drinking purposes in Sylhet district, Bangladesh, using entropy method, spatial autocorrelation index, and geostatistics. Environ Sci Pollut Res 24(34):26350–26374
Panaskar DB, Wagh VM, Pawar RS (2014) Assessment of groundwater quality for suitability of domestic and irrigation from Nanded Tehsil, Maharashtra, India. SRTMUs J Sci 4(1):71–83
Mukate S, Panaskar D, Wagh V, Muley A, Jangam C, Pawar R (2017) Impact of anthropogenic inputs on water quality in Chincholi industrial area of Solapur, Maharashtra, India. Groundw Sust Dev. https://doi.org/10.1016/j.gsd.2017.11.001
Pawar NJ, Pawar JB, Kumar S, Supekar A (2008) Geochemical eccentricity of ground water allied to weathering of basalts from the Deccan Volcanic Province, India: insinuation on CO2 consumption. Aquat Geochem 14(1):41–71
Beane JE, Turner CA, Hooper PR, Subbarao KV, Walsh JN (1986) Stratigraphy, composition and form of the Deccan basalts, Western Ghats, India. Bull Volcan 48(1):61–83
Taiwo AM, Adeogun AO, Olatunde KA, Adegbite KI (2011) Analysis of groundwater quality of hand-dug wells in peri-urban area of Obantoko, Abeokuta, Nigeria for selected physico-chemical parameters. Pac J Sci Technol 12(1):527–534
Dzik AJ (1989) Cerebrovascular disease mortality rates and water hardness in North Dakota. S D J Med 42(4):5–7
Stallard RF, Edmond JM (1983) Geochemistry of the Amazon: 2. The influence of geology and weathering environment on the dissolved load. J Geophys Res Oceans 88(C14):9671–9688
CPCB (2008) Guidelines for water quality management. Central Pollution Control Board, Delhi
Loizidou M, Kapetanios EG (1993) Effect of leachate from landfills on underground water quality. Sci Total Environ 128(1):69–81
Mukate S, Wagh V, Panaskar D, Jacobs JA, Sawant A (2019) Development of new integrated water quality index (IWQI) model to evaluate the drinking suitability of water. Ecol Indic 101:348–354
Hauser B (2001) Drinking water chemistry: a laboratory manual. CRC Press, Boca Raton
Wagh VM, Panaskar DB, Jacobs JA, Mukate SV, Muley AA, Kadam AK (2019) Influence of hydro-geochemical processes on groundwater quality through geostatistical techniques in Kadava river basin, Western India. Arab J Geosci 12(1):7
Wagh VM, Panaskar DB, Mukate SV, Aamalawar ML, Laxman Sahu U (2019) Nitrate associated health risks from groundwater of Kadava river basin, Nashik, Maharashtra, India. Hum Ecol Risk Assess Int J. https://doi.org/10.1080/10807039.2018.1528861
Comly HH (1945) Cyanosis in infants caused by nitrates in well water. J Am Med Assoc 129(2):112–116
Jeong CH (2001) Effect of land use and urbanization on hydrochemistry and contamination of groundwater from Taejon area, Korea. J Hydrol 253(1):194–210
Matthes G, Harvey JC (1982) The properties of water. Wiley, New York
Drever JI (1988) The geochemistry of natural waters, vol 437. Prentice Hall, Englewood Cliffs
Durov SA (1948) Natural waters and graphic representation of their composition. In: Dokl Akad Nauk SSSR, vol 59(3), pp 87–90
Chadha DK (1999) A proposed new diagram for geochemical classification of natural waters and interpretation of chemical data. Hydrogeol J 7:431–439. https://doi.org/10.1007/s100400050216
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Science 170(3962):1088–1090
Karanth KR (1987) Ground water assessment: development and management. Tata McGraw-Hill Education, New York
Singh AK, Mondal GC, Singh PK, Singh S, Singh TB, Tewary BK (2005) Hydrochemistry of reservoirs of Damodar River basin, India: weathering processes and water quality assessment. Environ Geol 48(8):1014–1028
Hounslow A (1995) Water quality data: analysis and interpretation. CRC press
Tiwari AK, Singh AK (2014) Hydrogeochemical investigation and groundwater quality assessment of Pratapgarh district, Uttar Pradesh. J Geol Soc India 83(3):329–343
Raju NJ, Patel P, Reddy BSR, Suresh U, Reddy TVK (2016) Identifying source and evaluation of hydrogeochemical processes in the hard rock aquifer system: geostatistical analysis and geochemical modeling techniques. Environ Earth Sci 75(16):1157
Datta PS, Tyagi SK (1996) Major ion chemistry of groundwater in Delhi area: chemical weathering processes and groundwater flow regime. J Geol Soc India 47:179–188
Zhu J (2014) Quantitative models for performance evaluation and benchmarking: data envelopment analysis with spreadsheets, vol 213. Springer, Berlin
Acknowledgements
The authors are thankful to Director, School of Earth Sciences for providing analytical facilities to complete the present research work. I am very much thankful to Ajaykumar Kadam for assisting me in data collection and analysis. Also, authors are very grateful to anonyms reviewers and editor of the journal for their meaningful suggestions and comments which help to improve the manuscript quality.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wagh, V.M., Mukate, S.V., Panaskar, D.B. et al. Study of groundwater hydrochemistry and drinking suitability through Water Quality Index (WQI) modelling in Kadava river basin, India. SN Appl. Sci. 1, 1251 (2019). https://doi.org/10.1007/s42452-019-1268-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-019-1268-8