Abstract
The increasing flexible and wearable electronic technology, demands cost effective and flexible energy devices which are safe to human body. Hence the benign biodegradable materials are becoming important class of materials for wearable energy devices. Starch is one such potential host renewable polymer which is abundant in nature and economical. Being a food ingredient, it is safe for human body. Its properties depends upon the amylose and amylopectin content in it and hence two different starches, corn (~ 27% amylose) and arrowroot (~ 15% amylose) are modified by sodium salt (NaClO4) and glutaraldehyde to develop flexible, transparent and free standing electrolyte membranes with high conductivity (> 10–3 S/cm). They have wide electrochemical stability window (> 2 V reaching upto 3.5 V) and low ESR. The relaxation time is of the order of µs and the cyclic voltammetry has indicated EDLC type of charge storage. At low frequency, the values of Cp/Cs are approaching to 1, indicating that all the available charges are polarizable and contributing to charge storage. The resonance frequency and frequency (f−45°) at which phase angle is −45°, are in kHz frequency range, i.e. the working frequency range is quite high. Electrolytes having corn starch (i.e. greater amount of amylose) have better performance on every electrochemical figure of merit.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The technology of wearable electronics is advancing at great pace and hence energy devices which are safe to human beings and cost effective are the demand of the time. That's why researchers are trying to synthesize basic components (electrode and electrolytes) of energy devices from natural/ renewable materials. The present paper deals with electrolytes for flexible and wearable devices. Ions, being bigger particle, need more space for moving and that's why the liquid electrolytes always give better conductivity in comparison to solid electrolytes, but many drawbacks like leakage, corrosion etc. are associated with them. Hence polymer electrolytes, which have solid physique and amorphous morphology, gained great popularity after the work of Armand and White in 1970s. Their work led to investigation of different combinations of polymers and additives (fillers and/or plasticizers) to achieve better electrochemical parameters along with making it cost effective, safe and environment friendly. In past few decades, polymers like Chitosan, Agar–agar, Cellulose etc. [1, 2]. which are benign to environment and human body, are being explored for the purpose. Unfortunately their conductivity and mechanical behavior are not good enough for commercial applications. In this series starch (a low cost and abundant renewable polymer) has also been tried [3, 4], because it fulfills many criteria, to be used as host, such as (i) salt acceptability, (ii) hanging moieties to support the ion transport, (iii) ease of film casting and (iv) abundance and low cost. Starches of different botanical origins have different ratio of amylose and amylopectin. Because of linear structure, amylose facilitates salt interaction in a better way in comparison to amylopectin where branched structure introduces steric hindrance in starch–salt interaction [5]. Such understanding of starch behavior indicates that starch with greater amylose content may be better for developing starch–salt complexed electrolyte system and hence a detailed investigation is required. Table 1[6] summarizes amylose content of some common starches. Among these common starches, amylose content is highest in corn whereas arrowroot has minimum amylose content, hence these two starches are selected for understanding the effect of host starch on electrolytic properties.
In polymer-salt electrolytes, salt selection is very important. Li salts, having best electrochemical properties, are supposed to be the best salts but their high cost, less abundance and flammable nature has prompted the scientists to look for the alternates. Hence different cations have been examined by researchers for replacing lithium. Their important electrochemical properties are summarized in Table 2 [7], which indicates that sodium and magnesium salts are the two possible alternates. Although their specific charge is quite less in comparison to Li but their electrode potential is comparable to Li and their abundance is tremendously high and they are cost effective also. Hence these two are being explored as possible cations for future devices. In present paper sodium perchlorate is selected as salt to synthesize flexible electrolyte membranes with corn and arrowroot starches.
2 Material preparation
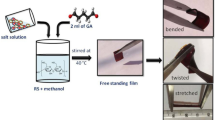
Corn starch (Jyoti chemie), Arrowroot starch (Jyoti chemie), Sodium perchlorate (Loba chemie), Glutaraldehyde (Merk) and Methanol (Fisher scientific) are used for material preparation. Materials have been synthesized using solution cast technique. Appropriate amount of corn starch and NaClO4 were separately dissolved in methanol and then mixed together, followed by addition of 2 ml glutaraldehyde (GA). This solution was stirred at 40 °C for 3 h till a homogeneous solution was obtained, which was then poured into a petri-dish and left for ambient drying. After 5–6 days free standing film was obtained. The same procedure was followed for arrowroot starch based systems also. For both the series starch/salt ratio is varied from 1:0.1 to 1:1. The prepared electrolytes, of both the series, are free standing, transparent and flexible as shown in Fig. 1a and b. This confirms that their physique is favorable for device fabrication. Here after CS-xE and AS-xE phrase will be used for corn starch based electrolytes and arrowroot based electrolytes respectively where x will represent the amount of salt for 1 g starch.
3 Techniques and formulas used for characterization
The prepared samples were cut into square pieces of size 10 × 10 mm2 with sharp blade. The impedance of the samples was measured by HIOKI HI tester LCR meter (3522 and 3532). The bulk resistance of sample has been estimated by nyquist plot and conductivity has been calculated using the formula.
where l and A is the thickness and area of the material, Z is the bulk resistance of the sample.
The polarization of the material and loss of the energy indicated by ε' and ε" and are estimated using the formula.
where Z' and Z" are the real and imaginary part of the impedance ω is angular frequency and C0 is the capacitance of empty measuring cell of electrode area A and sample dimension l given by
The capacitance is complex quantity and is represented as C(ω) = C' − jC" where C' and C" are the real and imaginary part of the capacitance and estimated using the following formula,
The series and parallel capacitance of the material calculated using the formula,
where f is frequency in Hz, Z' and Z" are real and imaginary part of impedance and ω is angular frequency.
Linear sweep voltammetry (LSV) is used to estimate the electrochemical stability window (ESW) of the material and has been performed using Agilent source measurement model number B2901A. Electrochemical behaviour of the system has been analysed using cyclic voltammetry and it is performed using CH instrument 600.
4 Results and discussion
The first and foremost important parameter for any electrolyte is its ionic transference number (tion) which indicates the ionic contribution to the total charge conduction. The tion estimated for all the prepared films were found to be greater than 0.98 indicating that they are predominantly ionic conductors and hence further analysis of the prepared materials were carried out. As the tion is measured using DC polarization technique with blocking electrodes and hence due to space charge polarization at electrode/electrolyte interfaces the value is little bit underestimated.
4.1 Nyquist plot study
Electrolytes are ion conducting materials hence dc method of conductivity measurement cannot be applied. For these materials AC impedance spectroscopy is used where imaginary and real impedance is monitored at different frequency and such plots (Z" Vs Z') are called nyquist plots. The Al/electrolyte/Al cell configuration is used for impedance analysis. The analysis was carried out for CS-xE and AS-xE samples with x varying from 0.1 to 1 in steps of 0.1, but in Figs. 2 and 3 the nyquist plots are shown for value of x from 0.6 to 1 only. For lower salt concentration (x ≤ 0.5) samples, resistance is high and systems show poor conductivity hence the data were neglected. The nature of nyquist plot gives an idea of equivalent circuit of the system [8]. In the frequency range, where resistance and capacitance both have comparable values, a semicircle is observed. It is the phenomenon which is obtained at higher frequency side. As we move towards the lower frequency side capacitive impedance starts dominating and hence only a spike is obtained.
In Figs. 2 and 3, moving from low salt concentration to higher salt concentration it is observed that semicircle part which are observable at low salt concentrations are missing at high salt concentration. Only spikes are present in nyquist plot for higher conducting materials whose position is shifted from origin [9]. The intersection point of nyquist plot at real Z axis shifts towards the lower value which indicates the amount of salt concentration is controlling the resistance of the system, and also that at higher salt concentration the behavior of Al/electrolyte/Al cell is dominated by capacitive impedance.
Warburg resistance (WR) is the measurement of resistive obstruction in transfer of ions from electrolyte to electrode [9]. It is related with diffusion (mass transfer) process. The smoother the ion transport the smaller is the warburg resistance. Warburg resistance is indicated by a slope change of spike in nyquist plot at higher frequency side. It is actually a line with 45°slope. In present series of systems, WR is present in all prepared samples and for higher salt concentration, its value is small. Figure 4 illustrates the position of Rb and Rb + W in the extended view of nyquist plot for CS-0.6E system [10]. Similarly WR is estimated for all starch/salt ratios of both the series of systems. The estimated values of WR are tabulated in Table 3. With increasing salt concentration WR is decreasing which may be attributed to increasing flexibility of starch based systems with increasing salt concentration [11] and hence better contact between electrode and electrolyte. The WR resistance is smaller for CS-xE in comparison to AS-xE for all values of x. This indicates that ion diffusion is much easier in corn based electrolyte which may be correlated with higher percentage of amylose in corn starch.
4.2 Effect of salt and temperature on conductivity
As seen in nyquist plots, the resistance of the prepared polymer-salt complex varies with salt concentration that means its conductivity is strongly affected by the salt concentration. Polymer–salt complex have very complex behavior where addition of salt not only gives the charge carrier to the system but simultaneously plasticizes the polymer matrix [12]. It should also be noted that addition of salt breaks the starch molecules to smaller weight molecules resulting in greater amorphicity [13] resulting in more salt interacting sites to accommodate the greater amount of salt. Performance of polymer-salt electrolytes are affected by salt concentration in two ways (i) salt concentration decides the number of charge carriers and (ii) it also give plasticization effect. A general understanding says that plasticization means increased amorphicity and hence increased mobility of charge carriers, but it is not always correct. According to Chandra et al. [14], increased amorphicity of polymeric system supports better dissociation of salt and hence increases the number of charge carriers, which may have adverse effect on the mobility. Figure 5 represents the conductivity variation with amount of salt for both the series i.e. CS + xE and AS + xE.
Generally for polymer-salt complexes, conductivity versus salt concentration curves have minimum between two maximum and the traditional explanation for such behavior is that the ion-pair formation effect arising from increased coulombic interaction whereas after certain higher salt concentration these ion pair are either re-dissociated or they form triplet ions with the available ions in neighbor [15]. While analyzing the salt concentration effect on conductivity of prepared electrolyte, it has been observed in both the series that initially conductivity rises with salt concentration, but after certain salt concentration (in case CS − xE at x = 0.4 and in AS − xE at x = 0.6) the fast increasing trend of σ vs salt concentration changes and now only a small effect of salt concentration can be seen. The behavior can be correlated with compromising effect of two phenomena (i) ion-association taking place at higher salt concentration which will decrease the conductivity and (ii) dominating effect of plasticizing behavior of salt in starch matrix which makes it more amorphous providing easy path for ion conduction [16, 17].
The stability of material with respect to ambient temperature is important for device fabrication. Dependence of σ on temperature has been monitored for CS + 1E and AS + 1E systems of both the series and graph are given in Fig. 6. Measurements were taken only up to 90 °C because after that sample is so flexible that holding it in spring loaded sample holder was not possible and the area and thickness started modifying. Within the studied temperature limits the curves are straight line indicating the Arrhenius type conductivity behavior [18]. Since the material is very flexible (amorphous) hence VTF type charge conduction may be prevailing but since the studied temperature range is very small it is difficult to comment. Hence no comment is made regarding the prevailing mechanism of temperature effect. Here the importance of the study is to know the effect of temperature on conductivity, which is found to be very small in ambient temperature variation range, indicating that the performance of device, fabricated using these electrolytes, will not vary too much with variation in ambient temperature. It proves the potentiality of the electrolytes for device fabrications.
4.3 Bode plot study
As discussed before, the straight spikes in nyquist plots are indication of capacitive behaviors [19] and as shown in Figs. 2 and 3 the plots are of almost straight line nature intersecting the Z' at lower value i.e. the charges are easily reaching to electrodes and getting accumulated there even though the charge storage electrodes are not used. Phase angle and frequency range, for which the phase angle remains above − 45°, are important indicative parameters regarding the potentiality of material for device application. Here also only high salt concentration systems (x > 0.5) are studied, because only for these systems nyquist plots have straight line nature and σ is high.
Bode phase and magnitude plots for CS − xE and AS − xE (x varying from 0.6 to 1) are shown in Figs. 7, 8, 9, and 10. The ideal capacitive behavior is indicated by phase angle equal to − 90°, hence for real systems, having the phase angle closer to − 90° is preferred. The values of phase angle at lower frequency side are − 75° (CS-1E) and − 72° (AS − 1E) and it is maintained up to 785 Hz and 295 Hz for Corn and Arrowroot starch based systems respectively. At higher side of frequency, phase angle is positive which is due to pseudo inductive effect of the system [20]. The working frequency range for which capacitive nature is dominated, is an important parameter and frequency (f-45°) at which the phase angle is − 45° is considered for comparative study of different materials [21]. For CS − 1E and AS − 1E, f−45° is ~ 13 kHz and ~ 12 kHz respectively. Generally this range is found to be from few Hz or mHz, but in the present system it is of the order of kHz which is much higher in comparison to other reported systems [22] i.e. the present electrolyte can be used for higher frequency applications. The f-45˚ for the corn starch based system is greater than that of arrowroot starch based system and the phase angle also follow the same trend indicating that corn starch based system is more flexible hence faster orientation of charge carriers are possible. This can be correlated with higher content of amylose in corn starch.
The Bode magnitude plot for the system of both the series are given in Figs. 9 and 10. The plot shows a minimum at a particular frequency, which is the frequency at which phase angle changes its sign and is designated as self resonance frequency (fr). fr is the frequency where capacitive and inductive nature vanishes and after which inductive nature come into picture. At this frequency impedance is purely resistive. Below the self resonance frequency the capacitive part is dominated and stores the charge efficiently. The self resonance frequencies for CS − 1E and AS − 1E systems are 206 kHz and 260 kHz respectively, which are quite high.
4.4 Analysis of capacitive properties
Literature survey indicates that capacitive application are presented in different sets (such as Cp and Cp/Cs as a function of frequency and variation of Cs and Cp as a function of Z') to retrieve specific information. The variation of C' (or Cp) with frequency for both the series are shown in Figs. 11 and 12. Within the studied frequency range the value of C' has an increasing trend, indicating that a better value of C' can be obtained if frequency beyond the experimental limit is explored. The variation of C" with frequency for both systems is shown in Figs. 13 and 14. The C" passes through a maximum at a particular frequency due to the resistive and capacitive components present in the system. The resistive component related with ohmic loss whereas capacitive is related with energy storage. The frequency (f0), at which maximum occur, gives the relaxation time (τ = 1/f0) of the systems. The relaxation time (τ) is defined as the time required to dissipate all the energy from the system [23]. This relaxation is combined effect of two processes i) diffusion of charge carriers from electrolyte to electrode and ii) movement of ions to the deepest pore of electrode. Since here specific porous electrodes are not used hence the estimated time is basically associated with the diffusion of ions in the electrolytes [24]. The values of τ are tabulated in Table 4 to indicate the effect of salt concentration on it. The relaxation time observed for CS + 1E is 70 µs which is smaller in comparison to AS + 1E system which is 75 µs, which indicates that higher content of amylose facilitates the ion transport. The observed relaxation times are quite small in comparison to data reported in literature for other systems, indicating faster ion transport in synthesized systems.
Cp is parallel capacitance or usable capacitance where as Cs is the total capacitance of the system. The value of Cp/Cs for ideal polarizable system should be 1 [25]. As the polarizability of the system depends upon the frequency of applied electric field hence it is important to monitor the variation of Cp/Cs as function of frequency. The variation of Cp/Cs with frequency is shown in Figs. 15 and 16 for the system of both the series of systems. The maximum value of Cp/Cs for different salt concentration varies but the highest conducting samples have the value 0.98 for both the systems, which is quite high confirming that all the available charges will contribute to charge storage process.
The C-R diagram i.e. the plot between the utilizable capacitance (Cp or C') and real part of impedance gives the information about achievable capacitance and the equivalent series resistance.. C–R diagram for studied systems are given in Figs. 17 and 18. The sudden drop of Cp shows the good contact of electrode and electrolyte. In the logarithmic scale presentation of Cp vs Z' curve, the line parallel to Z' axis gives the value of capacitance and the extrapolated curve intersecting the Z' axis gives the ESR value of the system [26]. Cp and ESR value of the CS − 1E and AS − 1E are 1.29 µF and 6.34 Ω and 2.78 µF and 7.14 Ω respectively.
4.5 Linear sweep voltammetry (LSV) and Cyclic voltammetry (CV) analysis
The electrochemical stability window (ESW) is the potential range within which the electrolyte shows stable behavior and it can be used for device application [27] in that particular range. In case of supercapacitor it is the energy density (E = CV2/2) deciding factor. The ESW are obtained from LSV curve. The value of ESW is estimated for synthesized electrolytes and summarized in Tables 4 and 5. The LSV is monitored in the potential range − 3 V to + 3 V for both the systems. The constant current region indicates ESW of the system; it is the region where current is controlled by diffusion process of ions in lattice [28]. Literature survey indicates that although the aqueous electrolytes are very promising material but they fail at ESW front as their ESW is ≤ 1.5 V. For the present systems the ESW values are > 2 V for both the series at all studied starch/salt concentration and for corn starch it reaches ~ 3.5 V. The ESW value depends upon the salt concentration and type of starch. As the amylose content of the corn starch is higher and hence the association of salt with the corn starch will obviously higher than that of arrowroot starch, and because of higher association with starch its dissociation at electrode will require higher electric field and hence higher ESW is observed for corn starch based electrolytes. As the salt concentration is increased the probability of ions undergoing the redox reaction at electrode/ electrolyte interface will increase and hence the ESW value decreases with increasing salt concentration (Figs. 19 and 20).
The cyclic ability of the system has been checked by cyclic voltammetry measurements. The CV curves for CS-1E and AS-1E are given in Figs. 21 and 22 at different scan rates. Figures 23 and 24 show these CV curves for highest conducting electrolytes at fixed scan rate for different cycles. The value of capacitance at different scan rates have been estimated and plotted as a function of scan rate in Figs. 25 and 26. The value of C is almost constant in studied range of scan rate. The rectangular shape of CV indicates the systems exhibit EDLC type charge storage [29] and absence of any peak rules out the possibility of faradic nature. Figure 23 shows that there is no deviation in Corn + NaClO4 system even after 327 cycle (the studied number of cycles) but Arrowroot + NaClO4 based system have shown some problem after 50 cycles.
5 Conclusions
Using corn and arrowroot starches, transparent, flexible and free standing electrolyte membranes have been developed. Being synthesized using starch, which is a food ingredient for human being; these membranes are much safer in comparison to electrolytes having synthetic polymers as host. The ambient conductivity of Corn and Arrowroot starch based systems is found to be 1.00 × 10–2 S/cm and 5.60 × 10–3 S/cm respectively. The transference number is > 0.95 for systems of both the series, which confirms the ion conducting nature.
Temperature has nominal effect on conductivity in the ambient temperature variation range which indicates that devices, fabricated using these electrolytes, will have temperature independent performance. Phase angle were approaching to − 80° and f-45° is quite high (~ kHz), indicating the easy charge accumulation at electrode. The values of Cp/Cs are also approaching towards 1 confirming that all the available charges are participating in charge accumulation process. ESW for all the systems are > 2 V and for some systems it goes beyond 3 V, the ESR value is quite low and the relaxation time (τ) for systems of both the series lies in µs. CV study has shown that EDLC type of charge storage phenomenon is prevailing in the system. High ESW and low τ indicates the possibility of achieving high energy density and power density using these electrolyte membranes. The corn starch, which has higher amylose content, has shown better performance on every electrochemical technical parameter.
References
Osman Z, Ibrahim ZA, Arof AK (2001) Conductivity enhancement due to ion dissociation in plasticized chitosan based polymer electrolytes. Corbohydr Polym 44:167–173
Rapheal E, Avellaneda CO, Manzoli B, Pawlika AK (2010) Agar-based films for application as polymer electrolyte. Electrochim Acta 55:1455–1459
Tiwari T, Pandey K, Srivastava N (2011) Effect of Glutaraldehyde on electrical properties of arrowroot starch + nay electrolyte system. J Appl Polym Sci. https://doi.org/10.1002/app
Pawlicka A, Sabadini AC, Raphael E, Dragunski DC (2008) Ionic conductivity thermogravimetry measurements of starch-based polymeric electrolytes. Mol Cryst Liq Cryst 485(1):804–816
Ahmad KAS, Arof AK (2010) Conductivity studies of starch-based polymer electrolytes. Ionics 16:123–129
Chauhan JK (2017) An experimental investigation of corn and arrowroot starch based electrolyte systems. PhD Thesis submitted to Banaras Hindu University, Varanasi, India.
Kumar M (2014) Ion dynamic studies of few sodium and ammonium salt containing polymer electrolyte systems. PhD Thesis submitted to Banaras Hindu University, Varanasi, India
Pandey GP, Hashmi SA (2013) Ionic liquid 1-ethyl-3-methylimidazolium tetracyanoborate-ased gel polymer electrolyte for electrochemical capacitors. J Mater Chem A 1:3372–3378
Teoh KH, Lim C-S, Liew C-W, Ramesh S, Ramesh S (2015) Elecrical double-layer capacitors with corn starch-based biopolymer electrolytes incorporating silica as filler. Ionics 21:2061–2068
Pandey GP, Hashmi SA (2013) Ionic liquid 1-ethyl-3-methylimidazolium tetracyanoborate –based gel polymer electrolyte for electrochemical capacitors. J Mater Chem A 1:3372–3378
Aziz SB, Hamsan MH, Abdullah RM, Kadir MFZ (2019) A promising polymer blend electrolytes based on chitosan: methyl cellulose for EDLC application with high specific capacitance and energy density. Molecules 24:2503. https://doi.org/10.3390/molecules24132503
Kumar M, Tiwari T, Chauhan JK, Srivastava N (2014) Understanding the ion dynamics and relaxation behavior from impedance spectroscopy of NaI doped Zwitterionic polymer system. Mater Res Express 1:045003
Yadav M, Kumar M, Tiwari T, Srivastava N (2017) Wheat starch + NaI: a high conducting environment friendly electrolyte system for energy devices, WS + NaI ionics paper. Ionics 23:2871–2880
Chandra S (1981) Superionic solids: principles and applications. North-holl. Publ. Co, North-holland
Kumar M, Tiwari T, Srivastava N (2012) Electrical transport behaviour of bio-polymer electrolyte system: potato starch + ammonium iodide. Carbohydr Polym 88:54–60
Mohamad AA et al (2005) Electrical conductivities studies on PVA/PVP KOH alkaline solid polymer blend electrolyte. Ionics 11:418–422
Srivastava R, Chandra S (2002) Silica alcogel-based proton conducting solid-liquid electrolyte composite. Solid State Ionics 152–153:741–747
Selvasekarapandian S et al (2006) Conductivity and thermal behaviour of proton conducting polymer electrolyte based on poly N-vinyl pyrrolidone. Eur Polym 42:2672–2677
Kumar A et al (2005) Investigation on electrochemical supercapacitor using polypyrrole redox electrodes and PMMA based gel electrolyte. Eur J 41:1373–1379
Manzo SC et al (2012) Inductive effect on the fuel cell cathode impedance spectrum at high frequencies. J Fuel Cell Sci Technol 9(051002–1):051002–51008
Zhang G-Q, Zhao Y-Q, Tao F, Li H-L (2006) Electrochemical characteristics and impedance spectroscopy studies of nano-cobalt silicate hydroxide for supercapacitor. J Power Sources 161:723–729
Sheng K, Sun Y, Li C, Yuan W, Shi G (2012) Ultrahigh-rate supercapacitors based on electrochemically reduced grapheme oxide for ac line—filtering. Sci Rep. https://doi.org/10.1038/srep00247
Pandey GP, Rastogi AC, Westgate CR (2014) All-solid-state supercapacitor with poly 3,4-ethylenedioxythiophene-coated fiber paper electrodes and ionic liquid gel polymer electrolyte. J Power Sources 245:857–865
Misnon II, Mohd Zain NK, Abd AR, Vidyadharan B, Jose R (2015) Electrochemical properties of carbon from oil palm kernel shell for high performance supercapacitors. Electrochim Acta 174:78–86
Zhu J, Xu Y, Wang J, Lin J, Sun X, Mao S (2015) The effect of various electrolyte cations on electrochemical performance of polypyrrole/RGO based supercapacitor. Phys Chem Chem Phys 17:28666–28673
Kurzweil P (2004) ac Impedance spectroscopy—a powerful tool for the characterization of materials and electrochemical power sources. In: Proceeding: the 14 international seminor on double layer capacitors. Deerfield Beach, FL, USA, December 6–8.
Majid SR et al (2013) Super-capacitive electro-chemical performance of polymer blend gel polymer electrolyte (GPE) in carbon based electrical double-layer capacitor. Electrochim Acta 112:678–685
Selvasekarapandian S et al (2017) Mg-ion conducting blend polymer electrolyte based on poly(vinyl alcohol): poly (acrylonitrile) with magnesium perchlorate. Solid State Ionics 308:90–100
Majid SR et al (2011) Preparation and characterization of magnesium ion gel polymer electrolyte for application in electrical double layer capacitor. Electrochim Acta 57:91–97
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chauhan, J.K., Yadav, D., Yadav, M. et al. NaClO4 added, corn and arrowroot starch based economical, high conducting electrolyte membranes for flexible energy devices. SN Appl. Sci. 2, 899 (2020). https://doi.org/10.1007/s42452-020-2660-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-2660-0