Abstract
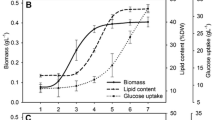
The fatty acid composition of the alga Chlorella saccharophila was investigated under different growth conditions. Using glucose as the sole carbon source, heterotrophically-grown Chlorella saccharophila produced a greater proportion of the polyunsaturated fatty acids (C18: 2 and C18: 3) than photosynthetic cultures, with linoleic acid (C18: 2) predominating. An unexpected discovery was the observation that at the lowest glucose concentration (2.5 gl−1) the lipid content of the algae increased to between 36–47% of the cell weight, depending on the temperature. At glucose concentrations of 5 g l−1 or more, the lipid content fell to 10–12% of the cell, although total fatty acid yield was higher due to higher biomass concentrations. Aeration of heterotrophic cultures promoted the production of unsaturated fatty acids compared to non-aerated cultures.
Similar content being viewed by others
References
Benemann, J. R., D. M. Tillet, & D. M. Weissman, 1987. Microalgae biotechnology. Tibtech. 5: 47–53.
Borowitzka, M. A., 1986. Microalgae as sources of fine chemicals. Microbiol. Sci. 3: 372–375.
Cohen, Z., A. Vonshak, & A. Richmond, 1988. Effect of environmental conditions on fatty acid composition of the red alga Porphyridium cruentum: correlation to growth rate. J. Phycol. 24: 328–332.
Dickson, L. G., R. A. Galloway, & G. W. Patterson, 1969. Environmentally-induced changes in the fatty acids of Chlorella. Plant Physiol. 44: 1413–1416.
Erwin, J. & K. Bloch, 1964. Biosynthesis of unsaturated fatty acids in microorganisms. Science. 143: 1006–1012.
Hansson, L. & M. Dostalek, 1988. Effect of culture conditions on mycelial growth and production of λ-linolenic acid by the fungus Mortierella rammanniana. Appl. Microbiol. Biotechnol. 28: 240–246.
Harrington, G. W. & G. G. Holz, 1968. The monoenoic and docosahexaenoic fatty acids of a heterotrophic dinoflagellate. Biochim. Biophys. Acta 164: 137–139.
Harris, P. & A. T. James, 1969. The effect of low temperatures on fatty acid biosynthesis in plants. Biochem. J. 112: 325–330.
Johns, M. R., C. K. Tan, & F. Chen, 1989. Heterotrophic microalgal production of chemicals. Proc. 8th Aust. Biotechnol Conf, Sydney, Australia. 542–545.
Metting, B. & J. W. Payne, 1986. Biologically active compounds from microalgae. Enzyme Microb. Technol. 8: 386–394.
Miller, R. L., H. E. Wickline & B. Richardson, 1971. Effects of heterotrophic and autotrophic growth conditions on the composition of Chlorella sorokiniana. J. Food Sci. 36: 774–777.
Morrison, W. R. & L. M. Smith, 1964. Preparation of fatty acid methyl esters and dimethyl acetals from lipids with boron trifluoride-methanol. J. Lipid Res. 5: 600–608.
Nagar-Legmannn, R. & P. Margalith, 1987. A comparative study of the lipid composition of yeasts with different fermentative capacities. Appl. Microbiol. Biotechnol. 26: 49–54.
Neidlemann, S. A., 1987. Effects of temperature on lipid unsaturation. Biotechnol. & Gen. Eng. Rev. 5: 245–268.
Nestel, P. J., 1988. Reassessment of the role of polyunsaturated fatty acids in cardiovascular disease. Food Technol. in Aust. 40: 225–257.
Nichols, B. W., 1965. Light induced changes in the lipids of Chlorella vulgaris. Biochim. Biophys. Acta 106: 274–279.
Patterson, G. W., 1969. Effect of culture temperature on fatty acid composition of Chlorella sorokiniana. Lipids 5: 597–600.
Piorreck, M., K. H. Baasch & P. Pohl, 1984. Biomass production, total protein, chlorophylls, lipids and fatty acids of freshwater and blue-green algae under different nitrogen regimes. Phytochemistry 23: 207–216.
Pohl, P. & F. Zurheide, 1982. Fat production in freshwater and marine algae. In H.A. Hoppe & T. Levring, (eds.), Marine Algae in Pharmaceutical Science, 2: Walter de Gruyter, Berlin: 65–79.
Radwan, S. S. & H. K. Mangold, 1980. Biochemistry of lipids in plant cell cultures. Adv. Biochem. Eng. 16: 109–133.
Seto, A., H. L. Wang, & C. W. Hesseltine, 1984. Culture conditions affect eicosapentaenoic acid content of Chlorella minutissima. JAOCS 61: 892–894.
Shimizu, S., H. Kawashima, Y., Shinmen, K. Akimoto, & H. Yamada, 1988. Production of eicosapentaenoic acid by Mortierella fungi. J. Am. Oil Chem. Soc. 65: 1455–1459.
Starr, R. C. & J. A. Zeikus, 1987. UTEX—The culture collection of algae at the University of Texas at Austin. J. Phycol. 23: 1–47.
Teshima, S., A., Kanazawa, N. Kamezaki, & H. Hirata, 1983. Effects of water temperature and salinity of eicosapentaenoic acid level of marine Chlorella. Bull. Jap. Soc. Scient. Fish 49: 805.
Wright, D. C., L. R. Berg, & G. W. Patterson, 1980. Effect of cultural conditions on the sterols and fatty acids of green algae. Phytochemistry 19: 783–785.
Yongmanitchai, W. & O. P. Ward, 1989. Omega-3 fatty acids: alternative sources of production. Process Biochem. 30: 117–125.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tan, C.K., Johns, M.R. Fatty acid production by heterotrophic Chlorella saccharophila . Hydrobiologia 215, 13–19 (1991). https://doi.org/10.1007/BF00005896
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00005896