Abstract
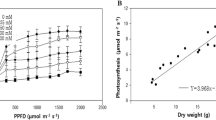
Cotton (Gossypium hirsutum L.) plants were grown in flowing-culture solutions containing 0%, 26% and 55% natural seawater under controlled and otherwise identical conditions. Leaf Na+ content rose to 360 mM in 55% seawater, yet the K+ content was maintained above 100 mM. The K+/Na+ selectivity ratio was much greater in the saline plants than in the control plants. All plants were healthy and able to complete the life cycle but relative growth rate fell by 46% in 26% seawater and by 83% in 55% seawater. Much of this reduction in growth was caused by a decreased allocation of carbon to leaf growth versus root growth. The ratio of leaf area/plant dry weight fell by 32% in 26% seawater and by 50% in 55 % seawater while the rate of carbon gain per unit leaf area fell by only 20% in 26% seawater and by as much as 66% in 55% seawater. Partial stomatal closure accounted for nearly all of the fall in the photosynthesis rate in 26% seawater but in 55% seawater much of the fall also can be attributed to non-stomatal factors. As a result of the greater effect of salinity on stomatal conductance than on CO2-uptake rate, photosynthetic water-use efficiency was markedly improved by salinity. This was also confirmed by stablecarbon-isotope analyses of leaf sugar and of leaf cellulose and starch. — Although non-stomatal photosynthetic capacity at the growth light was reduced by as much as 42% in 55% seawater, no effects were detected on the intrinsic photon yield of photosynthesis nor on the efficiency of photosystem II photochemistry, chlorophyll a/b ratio, carotenoid composition or the operation of the xanthophyll cycle. Whereas salinity caused in increase in mesophyll thickness and content of chloroplast pigments it caused a decrease in total leaf nitrogen content. The results indicate that the salinity-induced reduction in non-stomatal photosynthetic capacity was not caused by any detrimental effect on the photosynthetic apparatus but reflects a decreased allocation to enzymes of carbon fixation. — Rates of energy dissipation via CO2 fixation and photorespiration, calculated from gas-exchange measurements, were insufficient to balance the rate of light-energy absorption at the growth light. Salinity therefore would have been expected to cause the excess excitation energy to rise, leading to an increased nonradiative dissipation in the pigment bed and resulting increases in non-photochemical fluorescence quenching and zeaxanthin formation. However, no such changes could be detected, implying that salinity may have increased energy dissipation via a yet unidentified energy-consuming process. This lack of a response to salinity stress is in contrast to the responses elicited by short-term water stress which caused strong non-photochemical quenching and massive zeaxanthin formation.
Similar content being viewed by others
Abbreviations
- A:
-
net rate of CO2 uptake
- Ac :
-
calculated rate of CO2 uptake at constant pi
- Chl:
-
chlorophyll
- E:
-
rate of transpiration
- EPS:
-
epoxidation state of xanthophyll cycle components
- F, Fm :
-
fluorescence emission at the actual, full reduction of PSII reaction centers
- Fv :
-
variable fluorescence
- gs :
-
stomatal conductance to water vapor
- gw :
-
conductance to CO2transfer from intercellular spaces to chloroplasts
- NPQ:
-
non-photochemical fluorescence quenching
- pa, pi, pc :
-
atmospheric, intercellular and chloroplastic partial pressures of CO2
- PCRO:
-
photosynthetic carbon reduction and oxygenation cycle sum of the rate of carboxylation and oxygenation
- PFD:
-
photon flux density
- PSII:
-
photosystem II
- V+A+Z:
-
pool size of xanthophyll cycle components
- δ13C:
-
carbon-isotope composition
- (ϕPSII):
-
photon yield of PSII photochemistry at the actual reduction state in the light
References
Bilger, W., Björkman, O. (1990) role of the xantophyll cycle in photoprotection elucidated by measurements of light-induced absorbance changes, fluorescence and photosynthesis in leaves of Hedera canariensis. Photosynth. Res. 25, 173–185
Bilger, W., Björkman, O. (1991) Temperature dependance of violaxanthin de-epoxidation and non-photochemical fluorescence quenching in intact leaves of Gossypium hirsutum L. and Malva parviflora L., Planta 184, 226–234
Bilger, W., Schreiber, U. (1986) Energy-dependent quenching of dark-level chlorophyll fluorescence in intact leaves. Photosynth. Res. 10, 303–308
Bilger, W., Björkman, O., Thayer, S.S. (1989) Light-induced spectral absorbance changes in relation to photosynthesis and epoxidation state of xanthophyll cycle component in cotton leaves. Plant Physiol. 91, 542–551
Björkman, O. (1987) High-irradiance stress in higher plants and interaction with other stress factors. In: Progress in photosynthesis research, vol. IV, pp. 11–18, Biggins, J., ed. Martinus Nijhoff Publishers, Dordrecht, The Netherlands
Björkman, O., Demmig, B. (1987) Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77K among vascular plants of diverse origins. Planta 170, 489–504
Brooks, A., Farquhar, G.D. (1985) Effect of temperature on the CO2/O2 specificity of ribulose-1,5-bisphosphate carboxylase/ oxygenase and the rate of respiration in the light. Estimates from gas-exchange measurements of spinach. Planta 165, 387–406
Brugnoli, E., Björkman, O. (1992) Chloroplast movements in leaves: influence on chlorophyll fluorescence and measurements of light-induced absorbance changes related to ΔpH and zeaxanthin formation. Photosynth. Res., in press
Brugnoli, E., Hubick, K.T., von Caemmerer, S., Wong, S.C., Farquhar, G.D. (1988) Correlation between the carbon isotope discrimination in leaf starch and sugars of C3 plants and the ratio of intercellular and atmospheric partial pressures of carbon dioxide. Plant Physiol. 88, 1418–1424
Brugnoli, E., Lauteri, M. (1990) An evaluation of the effect of salinity on photosynthesis. In: Current research in photosynthesis, Vol. IV, pp. 741–744, Baltscheffsky, M., ed. Kluwer Academic Publishers, The Netherlands
Brugnoli, E., Lauteri, M. (1991) Effects of salinity on stomatal conductance, photosynthetic capacity, and carbon isotope discrimination of salt tolerant (Gossypium hirsutum L.) and saltsensitive (Phaseolus vulgaris L.) C3 non-halophytes. Plant Physiol. 95, 628–635
Chow, W.S., Ball, M.C., Anderson, J.M. (1990) Growth and photosynthetic responses of spinach to salinity: implication of K+ nutrition for salt tolerance. Aust. J. Plant Physiol. 17, 563–578
Cramer, G.R., Läuchli, A., Epstein, E. (1986) Effects of NaCl and CaCl2 on ion activities in complex nutrient solutions and root growth of cotton. Plant Physiol. 81, 792–797
Cramer, G.R., Epstein, E., Läuchli, A. (1990) Effect of sodium, potassium and calcium on salt stressed barley. I. Growth analysis. Physiol. Plant. 80, 83–88
Curtis, P.S., Läuchli, A. (1986) The role of leaf are development and photosynthetic capacity in determining growth of kenaf under moderate salt stress. Aust. J. Plant Physiol. 18, 553–565
Delane, R., Greenway, H., Munns, R., Gibbs, J. (1982) Ion concentration and carbohydrate status of the elongating leaf tissue of Hordeum vulgare growing at high external NaCl. I. Relationship between solute concentration and growth. J. Exp. Bot. 33, 557–573
Demmig, B., Winter, K., Krüger, A., Czygan, F.-C. (1987) Photoinhibition and zeaxanthin formation in intact leaves. A possible role of the xanthophyll cycle in the dissipation of excess light energy. Plant Physiol. 84, 218–224
Demmig, B., Winter, K., Krüger, A., Czygan, F.-C. (1988) Zeaxanthin and heat dissipation of excess light energy in Nerium oleander exposed to a combination of high light and water stress. Plant Physiol. 87, 17–24
Demmig-Adams, B. (1990) Carotenoids and photoprotection in plants: a role for the xanthophyll zeaxanthin. Biochim. Biophys. Acta 1020, 1–24
Evans, G.C. (1972) The quantitative analysis of plant growth. Studies in ecology, vol. 1, University of California Press, Berkeley and Los Angeles
Evans, J.R. (1983) Nitrogen and photosynthesis in the flag leaf of wheat (Triticum aestivum L.) Plant Physiol. 72, 297–302
Farquhar, G.D., Ehleringer, J.R., Hubick, K.T. (1989) Carbon isotope discrimination and photosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 40, 503–537
Flanagan, L.B., Jefferies, R.L. (1989) Effect of increased salinity on CO2 assimilation, O2 evolution and δ13C values of leaves of Plantago maritima L. developed at low and high NaCl levels. Planta 178, 377–384
Genty, B., Briantais, J.-M, Baker, N.R. (1989) The relationship between the quantum yield of photosynthetic electron transport and photochemical quenching of chlorophyll fluorescence Biochim. Biophys. Acta 990, 87–92
Isaac, R.A., Johnson, W.C. (1976) Determination of total nitrogen in plant tissue, using a block digestor. J. Assoc. Off. Analyt. Chem. 59, 98–100
Kobayashi, Y., Köster, S., Heber, U. (1982) Light scattering, chlorophyll fluorescence and state of the adenylate system in illuminated spinach leaves. Biochim. Biophys. Acta 682, 44–54
Krause, G.H., Weis, E. (1991) Chlorophyll fluorescence and photosynthesis: the basics. Annu. Rev. Plant Physiol. Plant Mol. Biol. 42, 313–349
Longstreth, D.J., Nobel, P.S. (1979) Salinity effects on leaf anatomy. Plant Physiol. 63, 700–703
Munns, R., Termaat, A. (1986) whole plant responses to salinity. Aust. J. Plant Physiol. 13, 143–160
Noctor, G., Rees, D., Young, A., Horton, P. (1991) The relationship between zeaxanthin, energy-dependent quenching of chlorophyll fluorescence, and trans-thylakoid pH gradient in isolated chloroplasts. Biochim. Biophys. Acta 1057, 320–330
Robinson, S.P., Downton, W.J.S., Mulhouse, J.A. (1983) Photosynthesis and ion content of leaves and isolated chloroplast of salt stressed spinach. Plant. Physiol. 73, 238–242
Schäfer, C., Björkman, O. (1989) Relationship between efficiency of photosynthetic energy conversion and chlorophyll fluorescence quenching in upland cotton (Gossypium hirsutum L.). Planta 178, 367–376
Schreiber, U., Neubauer, C. (1990) O2-dependent electron flow, membrane energization and the mechanism of non-photochemical quenching of chlorophyll fluorescence. Photosynth. Res. 25, 279–293
Seemann, J.R., Sharkey, T.D. (1986) Salinity and nitrogen effects on photosynthesis, ribulose-1,5-bisphosphate carboxylase and metabolite pool size in Phaseolus vulgaris L. Plant Physiol. 82, 555–560
Shennan, C., Hunt, R., Macrobbie, E.A.C. (1987) Salt tolerance in Aster tripolium L. I. The effect of salinity on growth. Plant Cell Environ. 10, 59–65
Terashima, I., Wong, S.C., Osmond, C.B., Farquhar, G.D. (1988) Characterisation of non-uniform photosynthesis induced by abscisic acid in leaves having different mesophyll anatomies. Plant Cell Physiol. 29, 385–395
Thayer, S.S., Björkman, O. (1990) Leaf xantophyll content and composition in sun and shade determined by HPLC. Photosynth. Res. 23, 331–343
von Caemmerer, S., Farquhar, G.D. (1981) Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153, 376–387
von Caemmerer, S., Evans, J.R. (1991) Determination of the average partial pressure of CO2 in chloroplasts from leaves of several C3 plants. Aust. J. Plant Physiol. 18, 287–305
West, D.W., Hoffman, G.J., Fisher, M.J. (1986) Photosynthesis, leaf conductance, and water relations of cowpea under saline conditions. Irrig. Sci. 7, 183–193
Yeo, A.R. (1983) Salinity resistance: physiologies and prices. Physiol. Plant. 58, 214–222
Yeo, A.R., Caporn, S.J.M., Flowers, T.J. (1985) The effect of salinity upon photosynthesis in rice (Oryza sativa L.); gas exchange by individual leaves in relation to their salt content. J. Exp. Bot. 36, 1240–1248
Author information
Authors and Affiliations
Additional information
* C.I.W.-D.P.B. Publication No. 1115, CNR-RAISA paper No. XXX
We thank Connie Shih for skilful assistance in growing plants and for conducting HPLC analyses and Barbara Mortimer for conducting the nitrogen analyses. Thanks are also due to C. Barry Osmond (now, Australian National University, Research School of Biological Sciences, Canberra) and Larry Giles of the Department of Botany, Duke University, Durham, N.C., for conducting carbonisotope analysis. E.B. was partially supported by the National Research Council of Italy, Special Project RAISA, Sub-Project No. 2. A Carnegie Institution Fellowship to E.B. is also gratefully acknowledged. This work was supported by grant No. 89-37-280-4902 of the Competitive Grant Program of the U.S. Department of Agriculture to O.B.
Rights and permissions
About this article
Cite this article
Brugnoli, E., Björkman, O. Growth of cotton under continuous salinity stress: influence on allocation pattern, stomatal and non-stomatal components of photosynthesis and dissipation of excess light energy. Planta 187, 335–347 (1992). https://doi.org/10.1007/BF00195657
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00195657